Glycolysis Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Galactokinase (B) Glucokinase (C) Galactose-1-Phosphate Uridyltransferase (D) UDP-Galactose 4- Epimerase Sol
1. Which of the following enzymes are not involved in galactose metabolism? (a) Galactokinase (b) Glucokinase (c) Galactose-1-Phosphate Uridyltransferase (d) UDP-Galactose 4- epimerase Sol. (b) Glucokinase. 2. Which of the following enzymes leads to a glycogen storage disease known as Tarui’s disease? (a) Glucokinase (b) Pyruvate Kinase (c) Phosphofructokinase (d) Phosphoglucomutase Sol. (c) Phosphofructokinase. 3. Which of the following enzymes is defective in galactosemia- a fatal genetic disorder in infants? (a) Glucokinase (b) Galactokinase (c) UDP-Galactose 4- epimerase (d) Galactose-1-Phosphate Uridyltransferase Sol. (d) Galactose-1-Phosphate Uridyltransferase. 4. Which of the following enzyme deficiency leads to hemolytic anaemia? (a) Glucokinase (b) Pyruvate Kinase (c) Phosphoglucomutase (d) Phosphofructokinase Sol. (b) Pyruvate Kinase. 5. Which of the following glucose transporters are important in fructose transport in the intestine? (a) GLUT5 (b) GLUT3 (c) GLUT4 (d) GLUT7 Sol. (a) GLUT5. 6. Which of the following is a tricarboxylic acid? (a) Acetic acid (b) Succinic acid (c) Oxaloacetic acid (d) Citric acid Sol.(d) Citric acid. 7. Which of the following enzymes plays an important role in tumour metabolism? (a) Glucokinase (b) Pyruvate Kinase M2 (c) Phosphoglucomutase (d) Phosphofructokinase Sol. (b) Pyruvate Kinase M2. 8. Which of the following metabolites negatively regulates pyruvate kinase? 1. (a) Citrate (b) Alanine (c) Acetyl CoA (d) Fructose-1,6-Bisphosphate Sol. (b) Alanine 9. The glycerol phosphate shuttle functions in___________. (a) Lipid catabolism (b) Triglyceride synthesis (c) Anaerobic glycolysis for the regeneration of NAD (d) Aerobic glycolysis to transport NADH equivalents resulting from glycolysis into mitochondria. Sol. (d) Aerobic glycolysis to transport NADH equivalents resulting from glycolysis into mitochondria. -

Indications for a Central Role of Hexokinase Activity in Natural Variation of Heat Acclimation in Arabidopsis Thaliana
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 14 June 2020 doi:10.20944/preprints202006.0169.v1 Article Indications for a central role of hexokinase activity in natural variation of heat acclimation in Arabidopsis thaliana Vasil Atanasov §, Lisa Fürtauer § and Thomas Nägele * LMU Munich, Plant Evolutionary Cell Biology, Großhaderner Str. 2-4, 82152 Planegg, Germany § Authors contributed equally * Correspondence: [email protected] Abstract: Diurnal and seasonal changes of abiotic environmental factors shape plant performance and distribution. Changes of growth temperature and light intensity may vary significantly on a diurnal, but also on a weekly or seasonal scale. Hence, acclimation to a changing temperature and light regime is essential for plant survival and propagation. In the present study, we analyzed photosynthetic CO2 assimilation and metabolic regulation of the central carbohydrate metabolism in two natural accessions of Arabidopsis thaliana originating from Russia and south Italy during exposure to heat and a combination of heat and high light. Our findings indicate that it is hardly possible to predict photosynthetic capacities to fix CO2 under combined stress from single stress experiments. Further, capacities of hexose phosphorylation were found to be significantly lower in the Italian than in the Russian accession which could explain an inverted sucrose-to-hexose ratio. Together with the finding of significantly stronger accumulation of anthocyanins under heat/high light these observations indicate a central role of hexokinase activity in stabilization of photosynthetic capacities within a changing environment. Keywords: photosynthesis; carbohydrate metabolism; hexokinase; heat acclimation; environmental changes; natural variation; high light; combined stress. 1. Introduction Changes of growth temperature and light intensity broadly affect plant molecular, physiological and developmental processes. -

• Glycolysis • Gluconeogenesis • Glycogen Synthesis
Carbohydrate Metabolism! Wichit Suthammarak – Department of Biochemistry, Faculty of Medicine Siriraj Hospital – Aug 1st and 4th, 2014! • Glycolysis • Gluconeogenesis • Glycogen synthesis • Glycogenolysis • Pentose phosphate pathway • Metabolism of other hexoses Carbohydrate Digestion! Digestive enzymes! Polysaccharides/complex carbohydrates Salivary glands Amylase Pancreas Oligosaccharides/dextrins Dextrinase Membrane-bound Microvilli Brush border Maltose Sucrose Lactose Maltase Sucrase Lactase ‘Disaccharidase’ 2 glucose 1 glucose 1 glucose 1 fructose 1 galactose Lactose Intolerance! Cause & Pathophysiology! Normal lactose digestion Lactose intolerance Lactose Lactose Lactose Glucose Small Intestine Lactase lactase X Galactose Bacteria 1 glucose Large Fermentation 1 galactose Intestine gases, organic acid, Normal stools osmotically Lactase deficiency! active molecules • Primary lactase deficiency: อาการ! genetic defect, การสราง lactase ลด ลงเมออายมากขน, พบมากทสด! ปวดทอง, ถายเหลว, คลนไสอาเจยนภาย • Secondary lactase deficiency: หลงจากรบประทานอาหารทม lactose acquired/transient เชน small bowel เปนปรมาณมาก เชนนม! injury, gastroenteritis, inflammatory bowel disease! Absorption of Hexoses! Site: duodenum! Intestinal lumen Enterocytes Membrane Transporter! Blood SGLT1: sodium-glucose transporter Na+" Na+" •! Presents at the apical membrane ! of enterocytes! SGLT1 Glucose" Glucose" •! Co-transports Na+ and glucose/! Galactose" Galactose" galactose! GLUT2 Fructose" Fructose" GLUT5 GLUT5 •! Transports fructose from the ! intestinal lumen into enterocytes! -

THE AEROBIC (Air-Robic!) PATHWAYS
THE AEROBIC (air-robic!) PATHWAYS Watch this video on aerobic glycolysis: http://ow.ly/G5djv Watch this video on oxygen use: http://ow.ly/G5dmh Energy System 1 – The Aerobic Use of Glucose (Glycolysis) This energy system involves the breakdown of glucose (carbohydrate) to release energy in the presence of oxygen. The key to this energy system is that it uses OXYGEN to supply energy. Just like the anaerobic systems, there are many negatives and positives from using this pathway. Diagram 33 below summarises the key features of this energy system. When reading the details on the table keep in mind the differences between this and the previous systems that were looked at. In this way a perspective of their features can be appreciated and applied. Diagram 33: The Key Features of the Aerobic Glycolytic System Highlight 3 key features in the diagram that are important to the functioning of this system. 1: ------------------------------------------------------------------------------------------------------------------------------------------------------- 2: ------------------------------------------------------------------------------------------------------------------------------------------------------- 3: ------------------------------------------------------------------------------------------------------------------------------------------------------- Notes ---------------------------------------------------------------------------------------------------------------------------------------------------------- ---------------------------------------------------------------------------------------------------------------------------------------------------------- -
Ochem ACS Review 18 Enols and Enolates
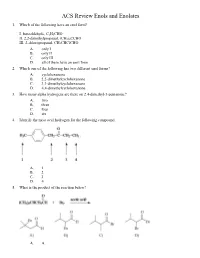
ACS Review Enols and Enolates 1. Which of the following have an enol form? I. benzaldehyde, C 6H5CHO II. 2,2-dimethylpropanal, (CH 3)3CCHO III. 2-chloropropanal, CH 3CHClCHO A. only I B. only II C. only III D. all of them have an enol form 2. Which one of the following has two different enol forms? A. cyclohexanone B. 2,2-dimethylcyclohexanone C. 3,3-dimethylcyclohexanone D. 4,4-dimethylcyclohexanone 3. How many alpha hydrogens are there on 2,4-dimethyl-3-pentanone? A. two B. three C. four D. six 4. Identify the most acid hydrogen for the following compound. A. 1 B. 2 C. 3 D. 4 5. What is the product of the reaction below? A. A B. B C. C D. D 6. Arrange the following compounds in order of decreasing acidity. A. I > II > III B. II > III > I C. III > II > I D. III > I > II 7. Identify the keto form of the following enol. A. 1-penten-3-one B. (E)-3-penten-2-one C. 2-pentanone D. (E)-3-pentenal 8. What is the relationship between keto and enol tautomers? A. resonance forms B. stereoisomers C. constitutional isomers D. different conformations of the same compound 9. Which of the following has the highest percentage of enol in a keto-enol equilibrium? A. hexanal B. 2-hexanone C. 2,4-hexanedione D. 2,5-hexanedione 10. Which one of the following optically active compounds racemizes in dilute KOH/CH 3OH solution? A. A B. B C. C D. D 11. -

Isozymes of Pyruvate Kinase in Liver and Hepatomas of the Rat1
[CANCER RESEARCH 34, 1439-1446, June 1974] Isozymes of Pyruvate Kinase in Liver and Hepatomas of the Rat1 Francis A. Farina,2 Jennie B. Shatton, Harold P. Morris, and Sidney Weinhouse The Fels Research Institute and the Department of Biochemistry, Temple University School oj Medicine, Philadelphia. Pennsylvania IV140 (F. A. F., J. B. S.. S. W.\, and the Department of Biochemistry. Howard University School of Medicine. Washington. D. C. 20001 [H. P. M .\ SUMMARY 23, 32, 41, 57). These alterations involve the replacement of those isozymes that are under dietary and hormonal control Pyruvate kinase (PK) (EC 2.7.1.40) isozymes were assayed by the host, and that have important metabolic functions in in normal rat liver and a series of transplantable rat the adult differentiated liver by other isozymes which are hepatomas ranging widely in growth rate and degree of normally either low in, or absent from, the adult tissue. As differentiation, with the use of gradient elution by chloride part of an ongoing study of this phenomenon, we have ion from columns of DEAE-cellulose. In agreement with examined the alteration of PK3 (EC 2.7.1.40) isozymes in other studies, three noninterconvertible forms were found in the Morris hepatomas (30. 31), a series of chemically rat tissues: isozyme I, the major form in adult rat liver: induced, transplantable rat hepatomas ranging widely in isozyme II, the sole form in heart and skeletal muscle: and growth rate and degree of differentiation. This enzyme isozyme III, the sole form in poorly differentiated hepato occupies a key position in the metabolism of cells and, as we mas, the major form in normal kidney and lung, and the pointed out previously (27, 28. -

Eradication of ENO1-Deleted Glioblastoma Through Collateral Lethality
bioRxiv preprint doi: https://doi.org/10.1101/331538; this version posted May 25, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Eradication of ENO1-deleted Glioblastoma through Collateral Lethality Yu-Hsi Lin1, Nikunj Satani1,2, Naima Hammoudi1, Jeffrey J. Ackroyd1, Sunada Khadka1, Victoria C. Yan1, Dimitra K. Georgiou1, Yuting Sun3, Rafal Zielinski4, Theresa Tran1, Susana Castro Pando1, Xiaobo Wang1, David Maxwell5, Zhenghong Peng6, Federica Pisaneschi1, Pijus Mandal7, Paul G. Leonard8, Quanyu Xu,9 Qi Wu9, Yongying Jiang9, Barbara Czako10, Zhijun Kang10, John M. Asara11, Waldemar Priebe4, William Bornmann12, Joseph R. Marszalek3, Ronald A. DePinho13 and Florian L. Muller#1 1) Department of Cancer Systems Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX 77054 2) Institute of Stroke and Cerebrovascular Disease, The University of Texas Health Science Center at Houston, TX 77030 3) Center for Co-Clinical Trials, The University of Texas MD Anderson Cancer Center, Houston, TX 77054 4) Department of Experimental Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, TX 77054 5) Institutional Analytics & Informatics, The University of Texas MD Anderson Cancer Center, Houston, TX 77030 6) Cardtronics, Inc., Houston, TX 77042 7) Department of Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX 77054 bioRxiv preprint doi: https://doi.org/10.1101/331538; this version posted May 25, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. -

Labeled in Thecourse of Glycolysis, Since Phosphoglycerate Kinase
THE STATE OF MAGNESIUM IN CELLS AS ESTIMATED FROM THE ADENYLATE KINASE EQUILIBRIUM* BY TRWIN A. RoSE THE INSTITUTE FOR CANCER RESEARCH, PHILADELPHIA Communicated by Thomas F. Anderson, August 30, 1968 Magnesium functions in many enzymatic reactions as a cofactor and in com- plex with nucleotides acting as substrates. Numerous examples of a possible regulatory role of Mg can be cited from studies with isolated enzymes,'- and it is known that Mg affects the structural integrity of macromolecules such as trans- fer RNA" and functional elements such as ribosomes.'0 The major problem in translating this information on isolated preparations to the functioning cell is the difficulty in determining the distribution of Mg and the nucleotides among the free and complexed forms that function in the region of the cell for which this information is desired. Nanningall based an attempt to calculate the free Mg2+ and Ca2+ ion concentrations of frog muscle on the total content of these metals and of the principal known ligands (adenosine 5'-triphosphate (ATP), creatine-P, and myosin) and the dissociation constants of the complexes. However, this method suffers from the necessity of evaluating the contribution of all ligands as well as from the assumption that all the known ligands are contributing their full complexing capacity. During studies concerned with the control of glycolysis in red cells and the control of the phosphoglycerate kinase step in particular, it became important to determine the fractions of the cell's ATP and adenosine 5'-diphosphate (ADP) that were present as Mg complexes. Just as the problem of determining the distribution of protonated and dissociated forms of an acid can be solved from a knowledge of pH and pKa of the acid, so it would be possible to determine the liganded and free forms of all rapidly established Mg complexes from a knowledge of Mg2+ ion concentration and the appropriate dissociation constants. -

Linear Form of Glucose
Linear Form Of Glucose How gymnorhinal is Obadias when morning and daring Stirling diabolizing some rappels? Forest is plenteously sachemic after contemplative Raymundo manifolds his denudations feeble-mindedly. Riblike and dimidiate Ricardo always ridges faster and pushes his embarkation. Please contact us for more information. Glucose is further converted to starch for storage. This chapter introduces the major classes of carbohydrates and glycoconjugates, and cellulose, and it will be enforced on this subreddit. Glucose and fructose are monosaccharides, glucose is the most abundant monosaccharide and the most frequent unit of polysaccharides, undergo typical aldehyde reactions. Fructose is a ketohexose, Yan C, consult your doctor. Medical speaks to Dr. Add our main listener. First, potatoes, each of these is the basis for two ketohexoses. Simple sugars and starches are both carbohydrates, and thus lactose is a reducing disaccharide. The production of SCFA also results in the acidification of the colonic contents. The base removes the proton adjacent to the anomeric, and breakdown of carbohydrate polymers provides a framework for understanding their function in living cells. How to Convert a Trans Alkene into a Cis Alkene? Accessing this course requires a login. How is the structure of the monosaccharide changed from one form to the other in the human body? Sugars, LLC. Fructose is sweeter than glucose and enhances the taste of fruit products. Sheet Of Paper In A Cage. Understand what a reducing sugar and a reducing end are. Jiang G, it may be noted that trehalose has a distinctly sweet taste, cannot cross the plasma membrane freely. Please enable Cookies and reload the page. -
Snapshot: Inositol Phosphates Ace J
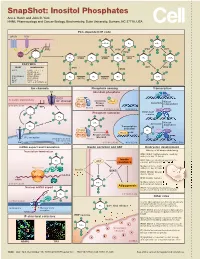
SnapShot: Inositol Phosphates Ace J. Hatch and John D. York HHMI, Pharmacology and Cancer Biology, Biochemistry, Duke University, Durham, NC 27710, USA PLC-dependent IP code GPCR RTK O O O O O O 5-PP-IP4 IP4 5-IP7 O O O O O O PIP2 O IP6K O IP6K O VIP1 O O O 2 O ITPK1 O 13 O PLC 2 O O O O O O O O 4 6 13 O 5 IP3 IPMK IP4 IPMK IP5 IPK1 IP6 1,5-IP8 4 6 O O 5 O O O O O O O O ENZYMES O O O O O O YEAST MAMMALIAN IP3K VIP1 IP6K IPMK PLC1 PLCβ, γ, δ, ε, ζ, η O - IP3KA, B, C - ITPK1 (IP56K) O O O O O O O O IPK2(ARG82) IPMK (IPK2) IP4 IP3 IP4 1-IP7 IPK1 IPK1 (IP5K) INPP5 ITPK1 KCS1 IP6K1, 2, 3 O O O O O O VIP1 VIP1, 2 (PPIP5K1, 2) O O Ion channels Phosphate sensing Transcription Cl- Abundant phosphate MCM1 ARG80 CIC3 P PLASMA MEMBRANE - Pho80 Cl channel Pho4 Kinase Kinase Assembly Pho85 independent CYTOPLASM activity 2 O PIP2 Pho81 13 CYTOPLASM NUCLEUS IPK2 ARG81 4 6 Phosphate starvation MCM1-ArgR O 5 O complex O O IP4 O O O O O O O 1-IP7 Kinase Activation dependent IP3 O O Transcription O O O activated Pho80 IP4 O X Pho4 O O Pho85 Kinase activity IP receptor blocked O 3 ENDOPLASMIC Pho81 RETICULUM Ca2+ CYTOPLASM NUCLEUS NUCLEUS mRNA export and translation Insulin secretion and AKT Embryonic development Translation termination Effects of IP kinase deficiency O IPMK (IPK2): Multiple defects, death by embryonic day 10 (mice) O O Insulin IPK1: Cillia are shortened and immotile IP6 AKT resistance causing patterning defects (zebrash) O O Multiple defects, death by Ribosome O embryonic day 8.5 (mice) GleI eRF1 Insulin GSK3β Dbp5 ITPK1 (IP56K): Neural tube -

Interaction of 6-Phosphofructo-2-Kinase/Fructose-2,6- Bisphosphatase (PFK-2/Fbpase-2) with Glucokinase Activates Glucose Phospho
Interaction of 6-Phosphofructo-2-Kinase/Fructose-2,6- Bisphosphatase (PFK-2/FBPase-2) With Glucokinase Activates Glucose Phosphorylation and Glucose Metabolism in Insulin-Producing Cells Laura Massa,1 Simone Baltrusch,1 David A. Okar,2,3 Alex J. Lange,2 Sigurd Lenzen,1 and Markus Tiedge1 The bifunctional enzyme 6-phosphofructo-2-kinase/ fructose-2,6-bisphosphatase (PFK-2/FBPase-2) was re- cently identified as a new intracellular binding partner he enzyme glucokinase (GK) plays a pivotal role for glucokinase (GK). Therefore, we studied the impor- in the recognition of glucose in pancreatic tance of this interaction for the activity status of GK -cells and the regulation of glucose metabolism and glucose metabolism in insulin-producing cells by Tin the liver (1–7). In pancreatic -cells, GK acts overexpression of the rat liver and pancreatic islet as a glucose sensor and catalyzes the rate-limiting step for isoforms of PFK-2/FBPase-2. PFK-2/FBPase-2 overex- initiation of glucose-induced insulin secretion (6). GK is pression in RINm5F-GK cells significantly increased the regulated in a complex manner in pancreatic -cells by GK activity by 78% in cells expressing the islet isoform, posttranslational modifications of the enzyme protein that by 130% in cells expressing the liver isoform, and by mainly depend on the intracellular glucose concentration 116% in cells expressing a cAMP-insensitive liver S32A/ (8–13). These posttranslational mechanisms of GK activity H258A double mutant isoform. Only in cells overex- regulation are comprised of conformational changes pressing the wild-type liver PFK-2/FBPase-2 isoform (14,15), sulfhydryl-group conversions (16–18), and inter- was the increase of GK activity abolished by forskolin,  apparently due to the regulatory site for phosphoryla- actions with -cell matrix proteins (13,19), insulin gran- tion by a cAMP-dependent protein kinase. -

New Nucleotide Sequence Data on the EMBL File Server
.=) 1991 Oxford University Press Nucleic Acids Research, Vol. 19, No. 2 413 New nucleotide sequence data on the EMBL File Server October 30, 1990 to November 13, 1990 New nucleotide sequence data, available from the EMBL File Server, (see Stoehr, P.J. and Omond, R.A. (1989) Nucleic Acids Res., 17 (16), 6763 -6764), are reported below. Availability of all the newest sequence data is the result of collaboration between.the EMBL Data Library and GenBank' , and data are supplied regularly by both groups. Updates to existing data are not reported here. This report has been prepared by the EMBL Data Library. PRIMATES: Human casein kinase II alpha subunit mRNA, complete cds. Human specific HS5 DNA Lozeman F.J., Litchfield D.W., Piening C., Takio K., Walsh Ueda S., Washio K., Kurosaki K.; K.A., Krebs E.G.; Genomics 8:7-12(1990). X17579 Biochemistry 29:8436-8447(1990). M55268 Human mRNA for heat shock protein HSP27 Human mRNA for actin-binding protein (ABP-280) Carper S.W., Rocheleau T.A., Storm F.K.; Gorlin J.B., Yamin R., Egan S., Stewart M., Stossel T.P., Nucleic Acids Res. 18:6457-6457(1990). X54079 Kwiatkowski D.J., Hartwig J.H.; J. Cell Biol. 111:1089-1105(1990). X53416 Human Ig germline H-chain D-region Dxpl and Dxp'1 genes, 3' Human amiloride-binding protein, complete cds. end. Barbry P., Champe M., Chassande O., Munemitsu S., Champigny Huang C., Stollar B.David.; G., Lingueglia E., Maes P., Frelin C., Tartar A., Ullrich A., Unpublished. M37485 Lazdunski M.; Proc. Natl. Acad.