Transfusion Medicine No
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hemolysis and Venous Thrombosis: Which Link? A
ISSN: 2378-3656 Rkiouak et al. Clin Med Rev Case Rep 2020, 7:329 DOI: 10.23937/2378-3656/1410329 Volume 7 | Issue 11 Clinical Medical Reviews Open Access and Case Reports ORIGINAL RESEARCH Hemolysis and Venous Thrombosis: Which Link? A. Rkiouak, PH.D1, I El Kassimi, MD2, N Sahel, MD2, M Zaizaa, MD2 and Y Sekkach, PhD1 Check for updates Internal Medicine A Department, Mohammed V Military Hospital Medical School of Rabat, Morocco *Corresponding author: Adil Rkiouak, PH.D., Department of Internal Medicine A, Mohammed V Military Hospital, Medical School of Rabat, Morocco, Tel: +212-66-179-44-04 The mechanism of antibody-mediated hemolysis is Abstract via phagocytosis or complement-mediated destruction The association hemolysis and venous thrombosis remains and can occur intravascular or extravascular. The intra- unknown to clinicians, despite our advances in comrehen- sion of pathophysiological bases. vascular mechanisms include direct cellular destruction via lysis, toxins, or trauma; fragmentation and oxida- Haemolysis, which is observed in multiple diseases, can affect all three components of Virchow’s triad. It is not sur- tion. prising that there is a link between haemolytic disorders and Multiple haemolytic disorders produce substantial thrombosis. intravascular haemolysis. Examples, the corpuscular We will try to clarify the main pro-thrombotic mechanisms hemolysis include PNH, extra-corpuscular haemolysis, during hemolysis through 3 clinical observations of deep ve- acquired (autoimmune haemolytic anaemia (AIHA , nous thrombosis in 3 main types of hemolytic pathologies, ) namely a case of paroxysmal nocturnal hemoglobinuria, thrombotic thrombocytopenic purpura (PTT)), as well thrombotic thrombocytopenic purpura and autoimmune as other diseases. These disorders are also associated anemia hemolytic. -

Clostridial Septicemia with Intravascular Hemolysis: a Case Report G
Henry Ford Hospital Medical Journal Volume 13 | Number 4 Article 4 12-1965 Clostridial Septicemia With Intravascular Hemolysis: A Case Report G. M. Mastio E. Morfin Follow this and additional works at: https://scholarlycommons.henryford.com/hfhmedjournal Part of the Life Sciences Commons, Medical Specialties Commons, and the Public Health Commons Recommended Citation Mastio, G. M. and Morfin, E. (1965) "Clostridial Septicemia With Intravascular Hemolysis: A Case Report," Henry Ford Hospital Medical Bulletin : Vol. 13 : No. 4 , 421-425. Available at: https://scholarlycommons.henryford.com/hfhmedjournal/vol13/iss4/4 This Article is brought to you for free and open access by Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Henry Ford Hospital Medical Journal by an authorized editor of Henry Ford Health System Scholarly Commons. Henry Ford Hosp. Med. Bull. Vol. 13, December 1965 CLOSTRIDIAL SEPTICEMIA WITH INTRAVASCULAR HEMOLYSIS A CASE REPORT G. M. MASTIC, M.D. AND E. MORFIN, M.D. In 1871 Bottini' demonstrated the bacterial nature of gas gangrene, but failed to isolate a causal organism. Clostridium perfringens, sometimes known as Clostridium welchii, was discovered independently during 1892 and 1893 by Welch, Frankel, "Veillon and Zuber.^ This organism is a saprophytic inhabitant of the intestinal tract, and may be a harmless saprophyte of the female genital tract occurring in the vagina in 4-6 per cent of pregnant women. Clostridial organisms occur in great numbers and distribution throughout the world. Because of this, they are very common in traumatic wounds. Very few species of Clostridia, however, are pathogenic, and still fewer are capable of producing gas gangrene in man. -
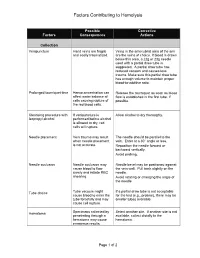
Factors Contributing to Hemolysis
Factors Contributing to Hemolysis Possible Corrective Factors Consequences Actions Collection Venipuncture Hand veins are fragile Veins in the antecubital area of the arm and easily traumatized. are the veins of choice. If blood is drawn below this area, a 22g or 23g needle used with a partial draw tube is suggested. A partial draw tube has reduced vacuum and causes less trauma. Make sure this partial draw tube has enough volume to maintain proper blood-to-additive ratio. Prolonged tourniquet time Hemoconcentration can Release the tourniquet as soon as blood affect water balance of flow is established in the first tube, if cells causing rupture of possible. the red blood cells. Cleansing procedure with If venipuncture is Allow alcohol to dry thoroughly. isopropyl alcohol performed before alcohol is allowed to dry, red cells will rupture. Needle placement Vein trauma may result The needle should be parallel to the when needle placement vein. Enter at a 30° angle or less. is not accurate. Reposition the needle forward or backward vertically. Avoid probing. Needle occlusion Needle occlusion may Needle bevel may be positioned against cause blood to flow the vein wall. Pull back slightly on the slowly and initiate RBC needle. shearing. Avoid rotating or changing the angle of the needle. Tube choice Tube vacuum might If a partial draw tube is not acceptable cause blood to enter the for the test (e.g., protime), there may be tube forcefully and may smaller tubes available. cause cell rupture. Hematoma Specimens collected by Select another site. If another site is not penetrating through a available, collect distally to the hematoma may cause hematoma. -

The Role of Methemoglobin and Carboxyhemoglobin in COVID-19: a Review
Journal of Clinical Medicine Review The Role of Methemoglobin and Carboxyhemoglobin in COVID-19: A Review Felix Scholkmann 1,2,*, Tanja Restin 2, Marco Ferrari 3 and Valentina Quaresima 3 1 Biomedical Optics Research Laboratory, Department of Neonatology, University Hospital Zurich, University of Zurich, 8091 Zurich, Switzerland 2 Newborn Research Zurich, Department of Neonatology, University Hospital Zurich, University of Zurich, 8091 Zurich, Switzerland; [email protected] 3 Department of Life, Health and Environmental Sciences, University of L’Aquila, 67100 L’Aquila, Italy; [email protected] (M.F.); [email protected] (V.Q.) * Correspondence: [email protected]; Tel.: +41-4-4255-9326 Abstract: Following the outbreak of a novel coronavirus (SARS-CoV-2) associated with pneumonia in China (Corona Virus Disease 2019, COVID-19) at the end of 2019, the world is currently facing a global pandemic of infections with SARS-CoV-2 and cases of COVID-19. Since severely ill patients often show elevated methemoglobin (MetHb) and carboxyhemoglobin (COHb) concentrations in their blood as a marker of disease severity, we aimed to summarize the currently available published study results (case reports and cross-sectional studies) on MetHb and COHb concentrations in the blood of COVID-19 patients. To this end, a systematic literature research was performed. For the case of MetHb, seven publications were identified (five case reports and two cross-sectional studies), and for the case of COHb, three studies were found (two cross-sectional studies and one case report). The findings reported in the publications show that an increase in MetHb and COHb can happen in COVID-19 patients, especially in critically ill ones, and that MetHb and COHb can increase to dangerously high levels during the course of the disease in some patients. -

Blood Banking/Transfusion Medicine Fellowship Program
DEPARTMENT OF PATHOLOGY AND LABORATORY MEDICINE BLOOD BANKING/TRANSFUSION MEDICINE FELLOWSHIP PROGRAM THIS NEW ONE-YEAR ACGME-ACCREDITED FELLOWSHIP IN BLOOD BANKING/TRANSFUSION MEDICINE OFFERS STATE OF THE ART COMPREHENSIVE TRAINING IN BLOOD BANKING, COAGULATION, APHERESIS, AND HEMOTHERAPY AT THE MEMORIAL HERMANN HOSPITAL (MHH)-TEXAS MEDICAL CENTER (TMC) FOR PEDIATRICS AND ADULTS. This clinically-oriented fellowship is ideal for the candidate looking for exceptional experience in hemotherapy decision-making and coagulation consultation. We have a full spectrum of medical and surgical specialties, including a level 1 trauma center, as well as a busy solid organ transplant service (renal, liver, pancreas, cardiac, and lung). Fellows will rotate through: Our unique hemotherapy service: This innovative clinical consultative service for the Heart and Vascular Institute (HVI) allows fellows to serve as interventional blood banking consultants at the bed side as part of a multidisciplinary care team; our patients have complex bleeding and coagulopathy issues. Therapeutic apheresis service: This consultative service provide 24/7 direct patient care covering therapeutic plasmapheresis, red blood cell (RBC) exchanges, photopheresis, plateletpheresis, leukoreduction, therapeutic phlebotomies, and other related procedures on an inpatient and outpatient basis. We performed approximately over 1000 therapeutic plasma exchanges and RBC exchanges every year. Bloodbank: The MHH-TMC reference lab is one of the largest in Southeast Texas. This rotation provides extensive experience in interpreting antibody panel reports, working up transfusion reactions, investigating blood compatibility/incompatibility issues, and monitoring component usage. Gulf Coast Regional Blood Center: This rotation provides donor exposure in one of the largest community blood donation centers in the US as well as cellular therapy and immunohematology training. -

Transfusion Medicine/Blood Banking
Transfusion Medicine/Blood Banking REQUIRED rotation This is a onetime rotation and is not planned by PGY level. Objective: The objective of this rotation is to teach fellows the clinical and laboratory aspects of Transfusion Medicine and Blood Banking as it impacts hematology/oncology. Goals: The goal of this rotation is to teach fellows blood ordering practices, the difference of type and screen and type and cross matches and selection of appropriate products for transfusion. Fellows will also learn to perform, watch type and screen red cell antibodies and will learn the significance of ABO, Rh and other blood group antigen systems briefly so as to understand the significance of red cell antibodies in transfusion practices. Fellows will learn the different types of Blood components, their indications and contraindications and component modification. e.g. Irradiated and washed products, and special product request and transfusions e.g. granulocyte transfusion, CMV negative products etc. Fellows will learn and become proficient in the laboratory aspects of autoimmune hemolytic anemia, coagulopathies and bleeding disorders in relation to massive transfusions and the laboratory aspects and management of platelet refractoriness. Fellows will learn some aspects of coagulation factor replacement for factor deficiencies and inhibitors. Activities: Fellows will rotate in the Blood Bank at UMC and learn to perform, observe and interpret standard blood bank procedures such as ABO Rh typing, Antibody Screen, Antibody Identification and Direct Antiglobulin Test (DAT). Fellows will also watch platelet cross matching for platelet refractoriness. Fellows will attend and present a few didactic lectures of about 40 minutes on Blood Bank related topics as they apply to hematology/oncology like blood group antigens, Blood Component therapy, Transfusion reactions, Autoimmune hemolytic anemia, Platelet immunology and HLA as it applies to transfusion medicine. -

A Suspected Case of Clostridium Perfringens Sepsis With
Uojima et al. Journal of Medical Case Reports (2019) 13:125 https://doi.org/10.1186/s13256-019-2023-x CASE REPORT Open Access A suspected case of Clostridium perfringens sepsis with intravascular hemolysis after transhepatic arterial chemoembolization: a case report Haruki Uojima1,2*, Mie Onoue2, Hisashi Hidaka2, Naohisa Wada2, Yoshiaki Tanaka2, Tomoyoshi Inoue2, Kousuke Kubota2, Takahide Nakazawa2, Akitaka Shibuya2 and Wasaburo Koizumi2 Abstract Introduction: Sepsis due to Clostridium perfringens, one of several clostridial species, is an important cause of massive intravascular hemolysis in patients with underlying malignancies. Chronic liver diseases, immunosuppression, and presence of malignancies were risk factors for Clostridium perfringens sepsis. Therefore, Clostridium perfringens sepsis should always be considered in patients presenting with liver damage after chemo- embolic therapy for hepatocellular carcinoma. This case report focuses on findings characteristic of an intravascular hemolysis due to Clostridium perfringens after transhepatic arterial chemoembolization. Case presentation: An 83-year-old Japanese man presented to our hospital because of a third recurrence of hepatocellular carcinoma. He had nonalcoholic steatohepatitis-related cirrhosis, and underwent radiofrequency ablation and transhepatic arterial chemoembolization therapy for hepatocellular carcinoma of S4/S8 and S2. He had a medical history of pancreatic carcinoma and underwent pylorus-preserving pancreaticoduodenectomy approximately 5 years ago. Because follow-up -

Current Practice in Transfusion Medicine April 2019
CURRENT PRACTICE IN TRANSFUSION MEDICINE APRIL 2019 Faculty/Speakers Luzmary Alverez Suzanne A. Arinsburg, DO Assistant Director, Blood Bank and Transfusion Services - Mount Sinai Hospital; Assistant Professor of Pathology - Icahn School of Medicine at Mount Sinai Scott Avecilla, MD, PhD Medical Director of Cellular Therapy and Assistant Medical Director of Transfusion Medicine, Department of Laboratory Medicine - Memorial Sloan Kettering Cancer Center Ian Bain, MD, PhD Ian received his medical training and PhD at the Albert Einstein College of Medicine in the Bronx, with his research work focusing on the transcriptional mechanisms underlying anergy in natural Regulatory T cells. He completed his training in Clinical Pathology, as well as subspecialty training in Molecular Genetic Pathology. Currently is a fellow in Transfusion Medicine and Apheresis at Yale-New Haven. He will be transitioning to a role as an Assistant Professor in the Department of Pathology at Mt Sinai Hospital/Icahn School of Medicine with a focus on transfusion medicine, apheresis and cell therapy. His academic interests center around mechanisms and sources of red cell alloimmunization, as well as the impact of novel immmunotherapies on autoantibody formation. Anna Burgos, MT(ASCP) SBB Senior Immunohematologist, Laboratory of Immunohematology and Genomics – New York Blood Center Carmelita Carrier, PhD Director, Fred H. Allen Laboratory of Immunogenetics, New York Blood Center Connie Cai Immunohematology, New York Blood Center Stephanie Dormesy Manager-Apheresis & -

Blood Bank I D
The Osler Institute Blood Bank I D. Joe Chaffin, MD Bonfils Blood Center, Denver, CO The Fun Just Never Ends… A. Blood Bank I • Blood Groups B. Blood Bank II • Blood Donation and Autologous Blood • Pretransfusion Testing C. Blood Bank III • Component Therapy D. Blood Bank IV • Transfusion Complications * Noninfectious (Transfusion Reactions) * Infectious (Transfusion-transmitted Diseases) E. Blood Bank V (not discussed today but available at www.bbguy.org) • Hematopoietic Progenitor Cell Transplantation F. Blood Bank Practical • Management of specific clinical situations • Calculations, Antibody ID and no-pressure sample questions Blood Bank I Blood Groups I. Basic Antigen-Antibody Testing A. Basic Red Cell-Antibody Interactions 1. Agglutination a. Clumping of red cells due to antibody coating b. Main reaction we look for in Blood Banking c. Two stages: 1) Coating of cells (“sensitization”) a) Affected by antibody specificity, electrostatic RBC charge, temperature, amounts of antigen and antibody b) Low Ionic Strength Saline (LISS) decreases repulsive charges between RBCs; tends to enhance cold antibodies and autoantibodies c) Polyethylene glycol (PEG) excludes H2O, tends to enhance warm antibodies and autoantibodies. 2) Formation of bridges a) Lattice structure formed by antibodies and RBCs b) IgG isn’t good at this; one antibody arm must attach to one cell and other arm to the other cell. c) IgM is better because of its pentameric structure. P}Chaffin (12/28/11) Blood Bank I page 1 Pathology Review Course 2. Hemolysis a. Direct lysis of a red cell due to antibody coating b. Uncommon, but equal to agglutination. 1) Requires complement fixation 2) IgM antibodies do this better than IgG. -

Approach to Anemia
APPROACH TO ANEMIA Mahsa Mohebtash, MD Medstar Union Memorial Hospital Definition of Anemia • Reduced red blood mass • RBC measurements: RBC mass, Hgb, Hct or RBC count • Hgb, Hct and RBC count typically decrease in parallel except in severe microcytosis (like thalassemia) Normal Range of Hgb/Hct • NL range: many different values: • 2 SD below mean: < Hgb13.5 or Hct 41 in men and Hgb 12 or Hct of 36 in women • WHO: Hgb: <13 in men, <12 in women • Revised WHO/NCI: Hgb <14 in men, <12 in women • Scrpps-Kaiser based on race and age: based on 5th percentiles of the population in question • African-Americans: Hgb 0.5-1 lower than Caucasians Approach to Anemia • Setting: • Acute vs chronic • Isolated vs combined with leukopenia/thrombocytopenia • Pathophysiologic approach • Morphologic approach Reticulocytes • Reticulocytes life span: 3 days in bone marrow and 1 day in peripheral blood • Mature RBC life span: 110-120 days • 1% of RBCs are removed from circulation each day • Reticulocyte production index (RPI): Reticulocytes (percent) x (HCT ÷ 45) x (1 ÷ RMT): • <2 low Pathophysiologic approach • Decreased RBC production • Reduced effective production of red cells: low retic production index • Destruction of red cell precursors in marrow (ineffective erythropoiesis) • Increased RBC destruction • Blood loss Reduced RBC precursors • Low retic production index • Lack of nutrients (B12, Fe) • Bone marrow disorder => reduced RBC precursors (aplastic anemia, pure RBC aplasia, marrow infiltration) • Bone marrow suppression (drugs, chemotherapy, radiation) -

Delayed Hemolytic Transfusion Reaction/Hyperhemolysis Syndrome in Children with Sickle Cell Disease
Delayed Hemolytic Transfusion Reaction/Hyperhemolysis Syndrome in Children With Sickle Cell Disease Julie-An M. Talano, MD*ʈ; Cheryl A. Hillery, MD*§ʈ; Jerome L. Gottschall, MD‡§ʈ; Diane M. Baylerian, BS, MT§ʈ; and J. Paul Scott, MD*§ʈ ABSTRACT. Objective. Alloimmunization in patients lower than it was at the time of original transfusion, with sickle cell disease (SCD) has a reported incidence of suggesting the hemolysis of the patient’s own RBCs in 5% to 36%. One complication of alloimmunization is addition to hemolysis of the transfused RBCs; a negative delayed hemolytic transfusion reaction/hyperhemolysis DAT and reticulocytopenia are often present. Severe (DHTR/H) syndrome, which has a reported incidence of complications including acute chest syndrome, conges- 11%. In patients with SCD, clinical findings in DHTR/H tive heart failure, pancreatitis, and acute renal failure syndrome occur approximately 1 week after the red were associated with DHTR/H syndrome in our patients. blood cell (RBC) transfusion and include the onset of DHTR/H in the pediatric sickle cell population is a seri- increased hemolysis associated with pain and profound ous and potentially life-threatening complication of RBC anemia. The hemoglobin (Hb) often drops below pre- transfusion. It is important to avoid additional trans- transfusion levels. In many reported adult cases, the di- fusions in these patients, if possible, because these may rect antiglobulin test (DAT) remains negative and no exacerbate the hemolysis and worsen the degree of new alloantibody is detected as the cause for these trans- anemia. DHTR/H syndrome must be included in the fusion reactions. -

Platelet Transfusion in the Real Life
4 Editorial Commentary Page 1 of 4 Platelet transfusion in the real life Pilar Solves1,2 1Blood Bank, Hematology Service, Hospital Universitari I Politècnic la Fe, Valencia, Spain; 2CIBERONC, Instituto Carlos III, Madrid, Spain Correspondence to: Pilar Solves. Blood Bank, Hematology Unit, Hospital Universitari I Politècnic La Fe, Avda Abril Martorell, 106, Valencia 46026, Spain. Email: [email protected]. Provenance and Peer Review: This article was commissioned by the editorial office,Annals of Blood. The article did not undergo external peer review. Comment on: Gottschall J, Wu Y, Triulzi D, et al. The epidemiology of platelet transfusions: an analysis of platelet use at 12 US hospitals. Transfusion 2020;60:46-53. Received: 06 April 2020. Accepted: 24 April 2020; Published: 30 June 2020. doi: 10.21037/aob-20-30 View this article at: http://dx.doi.org/10.21037/aob-20-30 Platelet transfusion is a common practice in thrombocytopenic period, between January 2013 and December 2016. patients for preventing or treating hemorrhages. Platelets The results provide a general view on current platelet are mostly transfused to patients diagnosed of onco- transfusion practice in US. A total of 163,719 platelets hematological diseases and/or undergoing hematopoietic representing between 3% and 5% of all platelets stem cell transplantation (1). With the aim to help transfused in the country, were transfused into 31821 physicians to take the most accurate decisions on platelet patients of whom 60.5% were males. More than 60% of transfusion, some guidelines have been developed (2-6). platelets were from single donor apheresis and 72.5% The scientific evidence supporting the recommendations were irradiated.