Submission Demonstrates That Flurbiprofen Lozenges Do Not Need to Be Restricted to Pharmacy Only Status Given;
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NOTE: the Following Are Shirley Ryan Abilitylab's Standard Charges As of Jan. 1, 2019. These Standard Charges Often Do Not
NOTE: The following are Shirley Ryan AbilityLab's standard charges as of Jan. 1, 2019. These standard charges often do not reflect what a patient or their insurance company/payer may be required to pay. Contact Patient Financial Services at 312-238-6039 for more information. Description Price 11-Deoxycortisol, Urine Random $531 12 Lead Ekg, Tracing Only $245 17 Hydroxycorticosteroids, Urine Timed $363 17 Hydroxyprogesterone, Serum $172 17 Ketosteroids, Urine Timed $273 17-Ketosteroids with Creatinine, Urine Random $273 2D Gait Analysis Charge(95999) $2,174 5' Nucleotidase, Serum $269 5.5 PDC PEDS CUFFED TRACH $226 5-HIAA-24 Hr Urine $99 A4566 Shoulder sling or vest design, abduction res $226 A4595 FES Electrode, each $38 A6545 Gradient Compression Wrap, Non-Elastic, Belo $171 ABO Rh Type $106 Above Knee Nylon Hose PR $83 Above Knee Suction Sheath $131 Above knee, for proximal femoral focal deficiency, $14,344 Above knee, molded socket, open end, SACH foot, en $10,751 Above knee, molded socket, single axis constant fr $10,935 Above knee, short prosthesis, no knee joint (""stu_L5210 $8,682 Above knee, short prosthesis, no knee joint (""stu_L5220 $7,429 ACAPELLA DH GREEN VIBRATORY PEP W/MOUTHPIECE DEVIC $181 Acetabuloplasty (27120) $4,493 Acetone, Serum $27 Acetylcholine Receptor Binding Antibody $405 Acid Phosphatase $66 Acid Phosphatase, Prostatic fraction $66 ACNE SURGERY MILIA CYSTS (10040) $530 Acorn Nebulizer $174 Activated PTT/Partial Thromboplastin Time $109 Active Wound Care > 20 cm Units $171 Active Wound Care/20 cm or < Units $171 Acupuncture with E-Stim. Each additional 15 minute $65 Acupuncture with E-Stim. -

Chewable Lozenge Formulation
Umashankar M S et al. Int. Res. J. Pharm. 2016, 7 (4) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Review Article CHEWABLE LOZENGE FORMULATION- A REVIEW Umashankar M S *, Dinesh S R, Rini R, Lakshmi K S, Damodharan N SRM College of Pharmacy, SRM University, Kattankulathur, India *Corresponding Author Email: [email protected] Article Received on: 11/02/16 Revised on: 13/03/16 Approved for publication: 28/03/16 DOI: 10.7897/2230-8407.07432 ABSTRACT Development of lozenges dated back to 20thcentury and is still remain popular among the consumer and hence it has continued commercial production. Lozenges are palatable solid unit dosage form administrated in the oral cavity. They meant to be dissolved in mouth or pharynx for its local or systemic effect. Lozenge tablets provide several advantages as pharmaceutical formulations however with some disadvantages. Lozenge as a dosage form can be adopted for drug delivery across buccal route, labial route, gingival route and sublingual route. Multiple drugs can also be incorporated in them for chronic illness treatments. Lozenge enables loading of wide range of active ingredients for oral systemic delivery of drugs. Lozenges are available as over the counter medications in the form of caramel based soft lozenges, hard candy lozenges and compressed tablet lozenges containing drugs for sore throat, mouth infection and as mouth fresheners. The rationale behind the use of medicated lozenges as one of the most favored dosage form for the delivery of antitussive drugs. This review focuses various aspects of lozenge formulation providing an insight to the formulation scientist on novel application of lozenge drug delivery system. -

Clinical Excellence Series Volume VI an Evidence-Based Approach to Infectious Disease
Clinical Excellence Series n Volume VI An Evidence-Based Approach To Infectious Disease Inside The Young Febrile Child: Evidence-Based Diagnostic And Therapeutic Strategies Pharyngitis In The ED: Diagnostic Challenges And Management Dilemmas HIV-Related Illnesses: The Challenge Of Emergency Department Management Antibiotics In The ED: How To Avoid The Common Mistake Of Treating Not Wisely, But Too Well Brought to you exclusively by the publisher of: An Evidence-Based Approach To Infectious Disease CEO: Robert Williford President & Publisher: Stephanie Ivy Associate Editor & CME Director: Jennifer Pai • Associate Editor: Dorothy Whisenhunt Director of Member Services: Liz Alvarez • Marketing & Customer Service Coordinator: Robin Williford Direct all questions to EB Medicine: 1-800-249-5770 • Fax: 1-770-500-1316 • Non-U.S. subscribers, call: 1-678-366-7933 EB Medicine • 5550 Triangle Pkwy Ste 150 • Norcross, GA 30092 E-mail: [email protected] • Web Site: www.ebmedicine.net The Emergency Medicine Practice Clinical Excellence Series, Volume Volume VI: An Evidence-Based Approach To Infectious Disease is published by EB Practice, LLC, d.b.a. EB Medicine, 5550 Triangle Pkwy Ste 150, Norcross, GA 30092. Opinions expressed are not necessarily those of this publication. Mention of products or services does not constitute endorsement. This publication is intended as a general guide and is intended to supplement, rather than substitute, professional judgment. It covers a highly technical and complex subject and should not be used for making specific medical decisions. The materials contained herein are not intended to establish policy, procedure, or standard of care. Emergency Medicine Practice, The Emergency Medicine Practice Clinical Excellence Series, and An Evidence-Based Approach To Infectious Disease are trademarks of EB Practice, LLC, d.b.a. -

Sore Throats
Pharyngitis Viruses cause most sore throats. When viruses infect your nose, $\/�P�1S1N(f F'ac+s: throat and sinuses, your body fightsback by making mucus. This helps Only about 15% of the people who wash out viruses. The mucus from your nose and sinuses drains into go to a doctor with a bad sore your throat. It can make your throat feel sore. Allergies, smoking, and throat have strep throat. You need air pollution can also lead to a sore throat. Some sore throats happen a test to tell for sure. You only need when stomach acid comes up into the throat. Yelling or speaking for a antibiotics if your test shows you long time can also make the throat sore. have strep throat. WJ.1affo l)O: Antibiotics don't work against viral infections. A sore throat • Drink more water. Honey and from a virus will get better on its own within a week or two. Antibiotics lemon in hot water or herbal teas won't make a sore throat go away any faster if it is caused by a virus. are good, too. Do not give honey Taking antibiotics when they are not needed may harm you by creating to children under 1. stronger germs. • Gargle with warm salt water. Talk with your health care provider about medicines that can help you • Suck on a hard candy, vitamin C feel better. For sore throats caused by allergies, your provider can help drop or throat lozenge. Do not give to young children. you figureout how to avoid the things that trigger your allergies. -

The Effect of Flurbiprofen on Postoperative Sore Throat And
Turk J Anaesth Reanim 2014; 42: 123-7 DOI: 10.5152/TJAR.2014.35693 The Effect of Flurbiprofen on Postoperative Sore Throat and Hoarseness After LMA-ProSeal Insertion: A Randomised, Clinical Trial Flurbiprofenin LMA- Proseal Uygulaması Sonrası Postoperatif Boğaz Ağrısı ve Ses Kısıklığı Üzerine Original Article AraştırmaOriginal / Özgün Etkisi: Randomize Klinik Çalışma Neslihan Uztüre, Ferdi Menda, Sevgi Bilgen, Özgül Keskin, Sibel Temur, Özge Köner Department of Anaesthesiology and Reanimation, Yeditepe University Faculty of Medicine, İstanbul, Turkey Objective: We hypothesized that flurbiprofen lozenges reduce the Amaç: Proseal laringeal maske ile ilişkili postoperatif boğaz ağrısı, ProSeal laryngeal mask airway (LMA) related symptoms of Post ses kısıklığı ve disfaji semptomlarını flurbiprofen pastilin azalttığı Operative Sore Throat (POST), hoarseness and dysphagia com- varsayımını gösterebilmek. pared to placebo lozenges. Yöntemler: Laringeal Maske (LMA) ile genel anestezi uygula- Abstract / Özet Abstract Methods: Eighty American Society of Anesthesiologists (ASA) I–II nacak 80 American Society of Anesthesiologists (ASA) I-II hasta patients undergoing general anaesthesia with LMA were included çalışmaya alındı. Prospektif, randomize, plasebo kontrollü, klinik in this prospective, randomized, placebo-controlled clinical and sin- ve tek merkezli (üniversite hastanesi) bir çalışma idi. Anestezi in- gle centre (university hospital) study. Group F received an 8.75 mg düksiyonundan 45 dakika önce Grup F’ye 8,75 mg flurbiprofen flurbiprofen -

How to Take Care of Your Sick Family
How to Take Care of Your Sick Family Molina Healthcare 24 Hour Nurse Advice Line 1-888-275-8750 TTY: 1-800-688-4889 Molina Healthcare Línea de TeleSalud Disponible las 24 Horas 1-866-648-3537 TTY: 1-866-833-4703 Important Phone Numbers 24 Hour Línea de TeleSalud Nurse Advice Line Disponible las 24 Horas 1-888-275-8750 1-866-648-3537 TTY: 1-800-688-4889 TTY: 1-866-833-4703 Family: Neighbor: Other: 1 Table of Contents How to Take Care of Your Sick Family Introduction...................................................2 Crying or Fussy Baby....................................4 Stomach Pain.................................................5 Headache........................................................8 Sore Throat...................................................11 Cough and Cold..........................................15 Painful Urination (Peeing).........................20 Ear Pain.........................................................23 Vomiting (Throwing Up)...........................26 Fever...............................................................30 Diarrhea........................................................34 Asthma or Trouble Breathing....................40 Constipation.................................................44 If you need this information in another language, large print, Braille, or in audio format please contact the number for Member Services on the back of your card. 2 3 Introduction help you decide if you or your family When your family does not feel well, you needs to see a health care want to help them provider. They can also let right away. This you know where your booklet gives you Provider’s office is. some quick tips on You can call our nurses 24 what you can do. hours a day, on any day. You should not use this booklet in place Nurse Advice Line La Línea de TeleSalud of what your health 1-888-275-8750 1-866-648-3537 care provider tells TTY: 1-800-688-4889 TTY: 1-866-833-4703 you. -

Preferred Drug List Mercy Care
Preferred Drug List Mercy Care Table of Contents *ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS*....................................................................5 *ALTERNATIVE MEDICINES*............................................................................................................................. 8 *AMINOGLYCOSIDES*......................................................................................................................................... 8 *ANALGESICS - ANTI-INFLAMMATORY*........................................................................................................ 8 *ANALGESICS - NONNARCOTIC*.....................................................................................................................11 *ANALGESICS - OPIOID*.................................................................................................................................... 12 *ANDROGENS-ANABOLIC*............................................................................................................................... 15 *ANORECTAL AGENTS*.....................................................................................................................................15 *ANTACIDS*..........................................................................................................................................................16 *ANTHELMINTICS*............................................................................................................................................. 16 *ANTIANGINAL -

Medicaid-Approved Preferred Drug List
New York Medicaid Medicaid-Approved Preferred Drug List Effective August 26, 2021 Legend In each class, drugs are listed alphabetically by either brand name or generic name. Brand name drug: Uppercase in bold type Generic drug: Lowercase in plain type AL: Age Limit Restrictions DO: Dose Optimization Program GR: Gender Restriction OTC: Over the counter medication available with a prescription. (Prescribers please indicate OTC on the prescription) PA: Prior authorization is required. Prior authorization is the process of obtaining approval of benefits before certain prescriptions are filled. QL: Quantity limits; certain prescription medications have specific quantity limits per prescription or per month. SP: Specialty Pharmacy ST: Step therapy is required. You may need to use one medication before benefits for the use of another medication can be authorized. MAT: Medication Assisted Therapy https://newyork.fhsc.com/providers/mat.asp Drug Name Reference Notes *ADHD/ANTI-NARCOLEPSY/ANTI- OBESITY/ANOREXIANTS* amphetamine-dextroamphet er oral capsule extended release 24 hour 10 mg, 15 mg, 5 Adderall XR DO; AL; QL mg amphetamine-dextroamphet er oral capsule extended release 24 hour 20 mg, 25 mg, 30 Adderall XR AL; QL mg amphetamine-dextroamphetamine oral Adderall DO; AL; QL tablet 10 mg, 12.5 mg, 15 mg, 5 mg, 7.5 mg amphetamine-dextroamphetamine oral Adderall AL tablet 20 mg, 30 mg atomoxetine hcl oral capsule Strattera DO; AL; QL caffeine citrate intravenous solution Cafcit caffeine citrate oral solution clonidine hcl er oral tablet extended -

Admissions Packet
Checklist for Admission Consent Forms *please read carefully and make sure all required forms are signed and completed* Name of Form Done Action Required Required Documents ☐ Must include with consent forms Admissions Record (pg. 1) Fill out completely ☐ *Please provide social security number* Privacy Practices Information (pg. 2-4) Keep Read HIPAA Acknowledgement (pg. 5) ☐ Fill out and sign Custodial Verification (pg. 6) ☐ Fill out, sign and attach applicable documentation Consent & Conditions (pg. 7-8) ☐ Fill out and sign Consent for Psychotherapy (pg. 9) ☐ Sign Seclusion & Restraint (pg. 10) ☐ Sign Individual Education Program (pg. 11) ☐ Fill out, sign and attach IEP if applicable Placement Disruption Agreement (pg. 12) ☐ Fill out and sign Permission for Adventure Outings (pg. 13) ☐ Check boxes, Initial and Sign Pivot Adventure (pg. 14) ☐ Sign Resident Rights Information (pg. 15) ☐ Fill out and sign Parent Financial Agreement (pg. 16) ☐ Fill out and sign Caseworker Financial Agreement (pg. 17) ☐ Fill out and sign Medication Reconciliation/Orders (pg. 18) ☐ Fill out completely and sign Immunizations (pg. 19) ☐ Sign & fill out or attach documentation Over the Counter Med Consent (pg. 20) ☐ Fill out and sign Resident Contact List (pg. 21) ☐ All individuals allowed to contact resident Student Record Request (pg. 22) ☐ Fill out as completely as possible Release of Information (pg. 23) Sign & fill out or make copies for all ☐ facilities/providers you desire to authorize Insurance Documentation (pg. 24) Fill out applicable sections - Please list Vision and ☐ *List Vision and Dental* Dental Insurances Resident Inventory (pg. 25) List all unperishable items (clothing, bedding, ☐ stuffed animals, etc.) the resident will be bringing Telehealth Informed Consent Form (pg. -

Randomised, Double-Blind, Placebo-Controlled Study of A
J Pharm Pharmaceut Sci (www.cspsCanada.org) 15(2) 281 - 294, 2012 Randomised, Double-Blind, Placebo-Controlled Study of a Single Dose of an Amylmetacresol/2,4-dichlorobenzyl Alcohol Plus Lidocaine Lozenge or a Hexylresorcinol Lozenge for the Treatment of Acute Sore Throat Due to Upper Respiratory Tract Infection Damien McNally,1 Adrian Shephard,2 Emma Field3 1 Ormeau Health Centre, Belfast, UK; 2 Professional Relations, Reckitt Benckiser, Slough, UK; 3 Global Medical Affairs, Reckitt Benckiser Healthcare, Hull, UK. Received, January 31, 2012; Revised, February 24, 2012; Accepted, April 1, 2012; Published, April 2, 2012. ABSTRACT - Purpose: Sore throat is a frequent reason for seeking medical care but few prescription options are available. Lozenges are effective in delivering active ingredients to the throat. This study was conducted to determine the analgesic efficacy of two lozenges one containing amylmetacresol (AMC)/2,4- dichlorobenzyl alcohol (DCBA) and lidocaine and one containing hexylresorcinol versus placebo in patients with acute sore throat due to upper respiratory tract infection (URTI). Methods: This was a multicentre, randomised, double-blind, parallel group, placebo-controlled study. In total, 190 patients were randomised 1:1:1 to a single dose of AMC/DCBA + lidocaine, hexylresorcinol or placebo lozenge. Subjective ratings of throat soreness, difficulty swallowing, swollen throat, numbing, and sore throat relief were obtained up to 2 hours post dose. Patient and investigator global ratings and a consumer questionnaire -

Quitnow Stop Smoking Medication Guide
Quitting Resources QuitNow Stop Smoking Medication Guide QUITNOW STOP SMOKING MEDICATION GUIDE Table of Contents page Intro 2 The B.C. Smoking Cessation Program 2 Nicotine Patches 3 – 5 Nicotine Gum 6 – 7 Nicotine Lozenge 8 – 10 Nicotine Inhaler 11 – 13 Nicotine Mouth Spray 14 – 16 Bupropion Sr (Zyban®) 17 – 18 Varenicline (Champix®) 19 - 20 1 Introduction This Medication Guide provides detailed information about various stop smoking aids that are approved for use in Canada to assist you with quitting smoking. While it is normal to experience withdrawal symptoms and cravings during the quit process, medications can significantly reduce the intensity of those symptoms and can double and even triple your chances of quit success. Note: This Medication Guide is for information purposes only and is not a substitute for the advice of a doctor or pharmacist. Medications Covered by the BC SmokinG Cessation ProGram In BC, the government’s Smoking Cessation Program helps you quit smoking by helping you with the cost of smoking cessation aids. Prescription stop smoking medications are covered as benefits under PharmaCare. Non-prescription nicotine replacement therapy products (i.e. patches and gum) are provided FREE to BC residents. Products covered under the Smoking Cessation Program include: 1) Two non-prescription (over-the-counter) Nicotine Replacement Therapy (NRT) products: a. nicotine gum (Thrive™); OR b. nicotine patch (Habitrol®) 2) Two prescription druGs: (See your doctor for prescription) a. bupropion (brand name Zyban®); OR b. varenicline (brand name Champix®) To learn more about how to access FREE or subsidized smoking cessation medications, visit the B.C. -
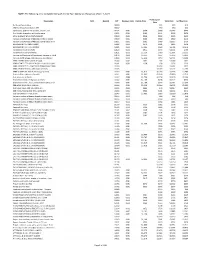
The Following Are a Complete Listing of Shirley Ryan Abilitylab Charges As of Jan
NOTE: The following are a complete listing of Shirley Ryan AbilityLab charges as of Jan. 1, 2021. Professional Description NDC DispVol CPT Revenue Code Facility Price Total Price Self Pay Price Price No Show/Cancellation 00000 $25 $25 $15 CPM-No Show/Cancellation $50 00002 $50 $50 $30 Injection(s), platelet rich plasma, any site, incl 0232T 0361 $954 $288 $1,242 $745 Fine Needle Aspiration without Imaging 10021 0361 $182 $111 $293 $176 ACNE SURGERY MILIA CYSTS (10040) 10040 0361 $338 $248 $586 $352 Incision and Drainage of Abscess, single or simple 10060 0361 $580 $380 $960 $576 Incision and Drainage of Abscess, complicated or m 10061 0361 $585 $680 $1,265 $759 I&D PILONID CYS; SMPL (10080) 10080 0361 $572 $291 $863 $518 I&D PILONID CYST; CPLX (10081) 10081 0361 $3,306 $493 $3,799 $2,279 FB removal in sub q-simple 10120 0361 $955 $376 $1,331 $799 FB removal in sub q-complex 10121 0361 $5,114 $780 $5,894 $3,536 Incision and Drainage of Hematoma, Seroma or Fluid 10140 0361 $4,755 $497 $5,252 $3,151 Incision and drainage complex, post-op infection 10180 0361 $6,011 $753 $6,764 $4,058 DEBR EXZ/INF SKIN; 10% BS (11000) 11000 0361 $992 $76 $1,068 $641 DEBRIDE INFECTED SKIN ADD-ON =<10% BS (11001) 11001 0361 $338 $38 $376 $226 DEBR NECROTIZ ST - GENITALS & PERINEUM (11004) 11004 $1,654 $1,654 $992 DEBR NECROTIZ STISS - ABD WALL (11005) 11005 $2,277 $2,277 $1,366 DEBR W OPEN FX; SKIN & SQ Charge (11010) 11010 0361 $1,812 $1,164 $2,976 $1,786 Debride Skin to Muscle, Open FX 11011 0361 $1,532 $1,318 $2,850 $1,710 Deb skin bone at FX site 11012