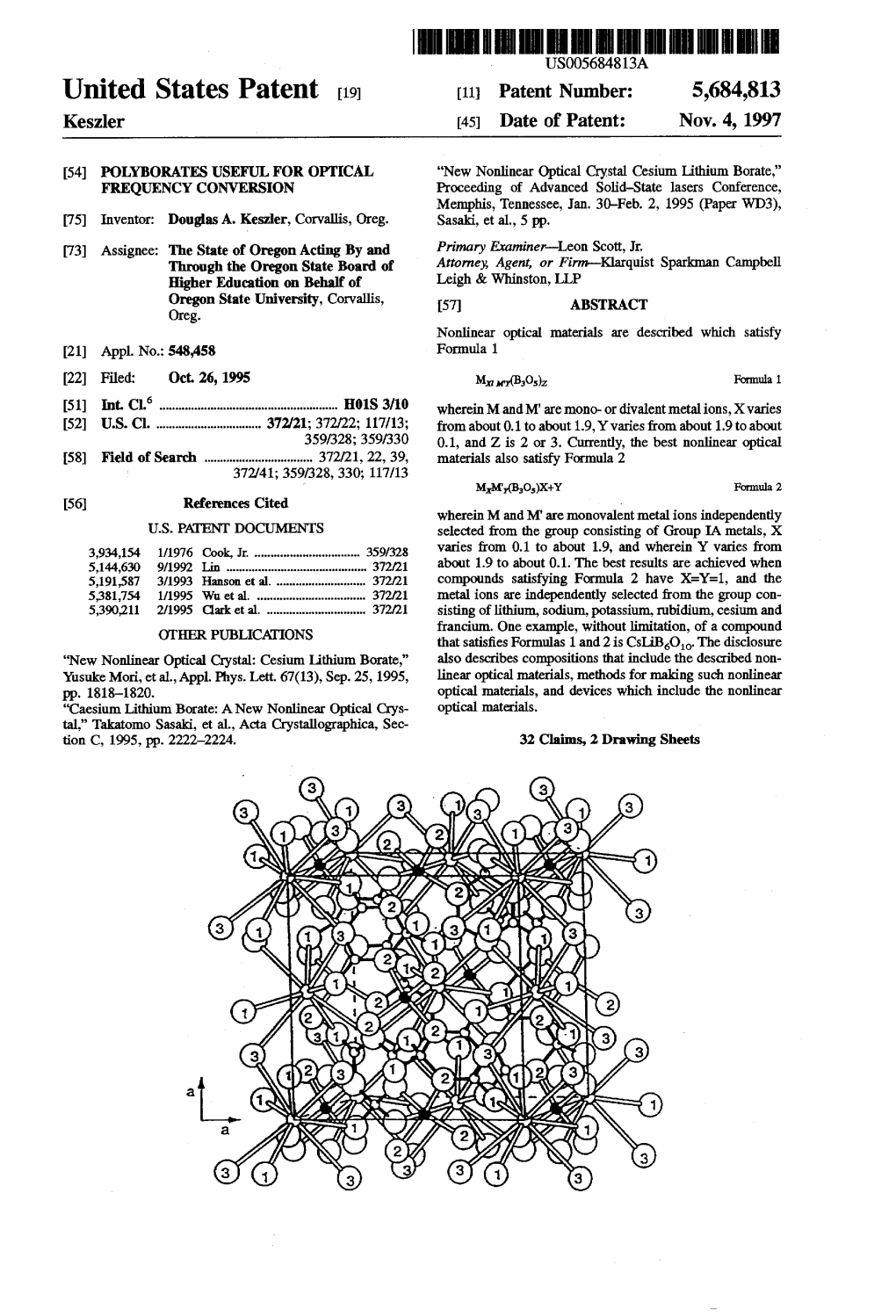
T. 26, 1995 Mary(Bos)Z Formula 1 (51) Int
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report on the Sonora Lithium Project
Report on the Sonora Lithium Project (Pursuant to National Instrument 43-101 of the Canadian Securities Administrators) Huasabas - Bacadehuachi Area (Map Sheet H1209) Sonora, Mexico o o centered at: 29 46’29”N, 109 6’14”W For 330 – 808 – 4th Avenue S. W. Calgary, Alberta Canada T2P 3E8 Tel. +1-403-237-6122 By Carl G. Verley, P. Geo. Martin F. Vidal, Lic. Geo. Geological Consultant Vice-President, Exploration Amerlin Exploration Services Ltd. Bacanora Minerals Ltd. 2150 – 1851 Savage Road Calle Uno # 312 Richmond, B.C. Canada V6V 1R1 Hermosillo, Mexico Tel. +1-604-821-1088 Tel. +52-662-210-0767 Ellen MacNeill, P.Geo. Geological Consultant RR5, Site25, C16 Prince Albert, SK Canada S6V 5R3 Tel. +1-306-922-6886 Dated: September 5, 2012. Bacanora Minerals Ltd. Report on the Sonora Lithium Project, Sonora, Mexico Date and Signature Page Date Dated Effective: September 5, 2012. Signatures Carl G. Verley, P.Geo. ____________________ Martin F. Vidal, Lic. Geo. Ellen MacNeill, P.Geo. Amerlin Exploration Services Ltd. - Consulting mineral exploration geologists ii Bacanora Minerals Ltd. Report on the Sonora Lithium Project, Sonora, Mexico Table of Contents 1.0 Summary ................................................................................................................................... 1 2.0 Introduction ............................................................................................................................... 3 3.0 Reliance on Other Experts ....................................................................................................... -

Mesoscopic Rydberg-Blockaded Ensembles in the Superatom Regime and Beyond
LETTERS PUBLISHED ONLINE: 12 JANUARY 2015 | DOI: 10.1038/NPHYS3214 Mesoscopic Rydberg-blockaded ensembles in the superatom regime and beyond T. M. Weber, M. Höning, T. Niederprüm, T. Manthey, O. Thomas, V. Guarrera†, M. Fleischhauer, G. Barontini† and H. Ott* The control of strongly interacting many-body systems of a Bose–Einstein condensate of 87Rb atoms. We first load the enables the creation of tailored quantum matter with complex condensate into a one-dimensional optical lattice with a spacing of properties. Atomic ensembles that are optically driven to a 532 nm, to suppress the axial movement of the atoms. We subse- Rydberg state provide many examples for this: atom–atom quently compress the atomic sample in the radial direction to reduce entanglement1,2, many-body Rabi oscillations3, strong its size below the blockade radius and empty all but three (or more) photon–photon interaction4 and spatial pair correlations5. lattice sites using a focused electron beam8–10 (Fig. 1a and Methods). In its most basic form Rydberg quantum matter consists of The atom number within the ensemble (N) can be adjusted between an isolated ensemble of strongly interacting atoms spatially 100 and 500 at a temperature of T D .3.5 0.5/ µK and the typical confined to the blockade volume—a superatom. Here we size of the sample is ≤3 µm in diameter (Fig. 1b). Our preparation demonstrate the controlled creation and characterization scheme is readily scalable to arrays of superatoms (Fig. 1d). of an isolated mesoscopic superatom by means of accurate After preparation we excite the atomic ensemble with a density engineering and excitation to Rydberg p-states. -

Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: a Review
resources Review Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review Laurence Kavanagh * , Jerome Keohane, Guiomar Garcia Cabellos, Andrew Lloyd and John Cleary EnviroCORE, Department of Science and Health, Institute of Technology Carlow, Kilkenny, Road, Co., R93-V960 Carlow, Ireland; [email protected] (J.K.); [email protected] (G.G.C.); [email protected] (A.L.); [email protected] (J.C.) * Correspondence: [email protected] Received: 28 July 2018; Accepted: 11 September 2018; Published: 17 September 2018 Abstract: Lithium is a key component in green energy storage technologies and is rapidly becoming a metal of crucial importance to the European Union. The different industrial uses of lithium are discussed in this review along with a compilation of the locations of the main geological sources of lithium. An emphasis is placed on lithium’s use in lithium ion batteries and their use in the electric vehicle industry. The electric vehicle market is driving new demand for lithium resources. The expected scale-up in this sector will put pressure on current lithium supplies. The European Union has a burgeoning demand for lithium and is the second largest consumer of lithium resources. Currently, only 1–2% of worldwide lithium is produced in the European Union (Portugal). There are several lithium mineralisations scattered across Europe, the majority of which are currently undergoing mining feasibility studies. The increasing cost of lithium is driving a new global mining boom and should see many of Europe’s mineralisation’s becoming economic. The information given in this paper is a source of contextual information that can be used to support the European Union’s drive towards a low carbon economy and to develop the field of research. -

Winter 2003 Gems & Gemology
Winter 2003 VOLUME 39, NO. 4 EDITORIAL _____________ 267 Tomorrow’s Challenge: CVD Synthetic Diamonds William E. Boyajian FEATURE ARTICLES _____________ 268 Gem-Quality Synthetic Diamonds Grown by a Chemical Vapor Deposition (CVD) Method Wuyi Wang, Thomas Moses, Robert C. Linares, James E. Shigley, Matthew Hall, and James E. Butler pg. 269 Description and identifying characteristics of Apollo Diamond Inc.’s facetable, single-crystal type IIa CVD-grown synthetic diamonds. 284 Pezzottaite from Ambatovita, Madagascar: A New Gem Mineral Brendan M. Laurs, William B. (Skip) Simmons, George R. Rossman, Elizabeth P. Quinn, Shane F. McClure, Adolf Peretti, Thomas Armbruster, Frank C. Hawthorne, Alexander U. Falster, Detlef Günther, Mark A. Cooper, and Bernard Grobéty A look at the history, geology, composition, and properties of this new cesium-rich member of the beryl group. 302 Red Beryl from Utah: A Review and Update James E. Shigley, Timothy J. Thompson, and Jeffrey D. Keith A report on the geology, history, and current status of the world’s only known occurrence of gem-quality red beryl. pg. 299 REGULAR FEATURES _____________________ 314 Lab Notes • Chrysocolla “owl” agate • Red coral • Coated diamonds • Natural emerald with nail-head spicules • Emerald with strong dichroism • High-R.I. glass imitation of tanzanite • Large clam “pearl” • Blue sapphires with unusual color zoning • Spinel with filled cavities 322 Gem News International • Comparison of three historic blue diamonds • Natural yellow diamond with nickel-related optical centers -

Ultraviolet Photoacoustic Remote Sensing Microscopy
Ultraviolet Photoacoustic Remote Sensing Microscopy by Nathaniel Haven A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Biomedical Engineering Department of Electrical and Computer Engineering University of Alberta © Nathaniel Haven, 2019 Abstract Cancer is currently one of the leading causes of death globally. In dealing with certain cancers, the recommended treatment is often surgery. In order to determine if the entirety of the cancerous mass has been removed, tumor resection margins are assessed after surgical resection, as it is difficult to differentiate cancerous from health tissue without micro-scale observation. Presently, the current gold-standard for tissue biopsy and tumor resection margin analysis is formalin-fixed paraffin-embedded hematoxylin & eosin (H&E) histology. Though, this process is both time consuming and labor intensive as well as being a significant burden on the healthcare economy. Unfortunately, there are currently no intraoperative techniques capable of reproducing reliable post-operative H&E histology output, this results in many patients having to undergo secondary surgeries, leading to unnecessary emotional and physical trauma for patients, as well as higher risks of worsened prognosis. Here we report the development of an ultraviolet excitation photoacoustic remote sensing microscopy system capable of producing images of cell nuclei within cell cultures and tissue samples. This was achieved in two iterations. The first iteration utilized a parabolic focusing element along with mechanical stage scanning to obtain images of sectioned HT1080 CAM tumors, as well as HeLa cell cultures, demonstrating the ability to observe individual cell nuclei with good comparison ii to H&E histology. -

The Use of Lithium to Prevent Or Mitigate Alkali-Silica Reaction in Concrete Pavements and Structures
The Use of Lithium to Prevent or Mitigate Alkali-Silica Reaction in Concrete Pavements and Structures PUBLIcatION NO. FHWA-HRT-06-133 MARCH 2007 Research, Development, and Technology Turner-Fairbank Highway Research Center 6300 Georgetown Pike McLean, VA 22101-2296 Foreword Progress is being made in efforts to combat alkali-silica reaction in portland cement concrete structures—both new and existing. This facts book provides a brief overview of laboratory and field research performed that focuses on the use of lithium compounds as either an admixture in new concrete or as a treatment of existing structures. This document is intended to provide practitioners with the necessary information and guidance to test, specify, and use lithium compounds in new concrete construction, as well as in repair and service life extension applications. This report will be of interest to engineers, contractors, and others involved in the design and specification of new concrete, as well as those involved in mitigation of the damaging effects of alkali-silica reaction in existing concrete structures. Gary L. Henderson, P.E. Director, Office of Infrastructure Research and Development Notice This document is disseminated under the sponsorship of the U.S. Department of Transportation in the interest of information exchange. The U.S. Government assumes no liability for its contents or use thereof. This report does not constitute a standard, specification, or regulation. The U.S. Government does not endorse products or manufacturers. Trade or manufacturers’ names appear herein only because they are considered essential to the objective of this manual. Quality Assurance Statement The Federal Highway Administration (FHWA) provides high-quality information to serve Government, industry, and the public in a manner that promotes public understanding. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0255278 A1 Bakke Et Al
US 20140255278A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0255278 A1 Bakke et al. (43) Pub. Date: Sep. 11, 2014 (54) METHODS TO RECOVER CESIUMOR (60) Provisional application No. 61/772,946, filed on Mar. RUBDIUM FROMISECONDARY ORE 5, 2013. (71) Applicant: Cabot Corporation, Boston, MA (US) Publication Classification (72) Inventors: Bart Bakke, The Woodlands, TX (US); (51) Int. Cl. Claude Deveau, Pinawa (CA) C22B 26/10 (2006.01) BO2C23/08 (2006.01) (73) Assignee: Cabot Corporation, Boston, MA (US) (52) U.S. Cl. CPC ................. C22B 26/10 (2013.01); B02C 23/08 (21) Appl. No.: 14/277.914 (2013.01) USPC ............ 423/202; 209/552; 209/10; 209/44.1; (22) Filed: May 15, 2014 241/24.1 (57) ABSTRACT Related U.S. Application Data A method to recover cesium, rubidium, or both from second (63) Continuation of application No. 14/191,468, filed on ary ore is described and involves using scans and sorting Feb. 27, 2014. techniques. Refined secondary ore is further described. : Si.23 C. c. c. g. WSg. ...c. 4 3 4. 3 - Chute or Moving Bett 2. Vibratory Pan ------------------------- -------...---------- -------------------------...--- --- - - -va-voom myr v. m- , 4-x-ray sensor r“s ; ry 5 image? - \,-- W---m i Signal Y 3. A. m t www.------------ - Processor & Dual Energy Beam --- ...i 4. - . NW --- \ - N-D - a3 ?y\l- 2 ww...w-xwr- . 6. High Speed Air Jet --A - 7 - 7-Separated Process Streams 8- Remote User interface Patent Application Publication Sep. 11, 2014 Sheet 1 of 4 US 2014/0255278 A1 Fig. -

Pegmatite Genesis: State of the Art
Eur. J. Mineral. 2008, 20, 421–438 Published online August 2008 Paper presented at the symposium “Granitic Pagmatites: the State of the Art”, Porto, May 2007 Pegmatite genesis: state of the art Wm. B. “Skip” SIMMONS* and Karen L. WEBBER Department of Earth and Environmental Sciences, University of New Orleans, New Orleans, LA 70148, USA *Corresponding author, e-mail: [email protected] Abstract: No one universally accepted model of pegmatite genesis has yet emerged that satisfactorily explains all the diverse features of granitic pegmatites. Genesis from residual melts derived from the crystallization of granitic plutons is favoured by most researchers. Incompatible components, fluxes, volatiles and rare elements, are enriched in the residual melts. The presence of fluxes and volatiles, which lower the crystallization temperature, decrease nucleation rates, melt polymerization and viscosity, and increase diffusion rates and solubility, are considered to be critical to the development of large crystals. A number of new concepts have shed light on problems related to pegmatite genesis. Cooling rates calculated from thermal cooling models demonstrate that shallow-level pegmatites cool radically more rapidly than previously believed. Rapid cooling rates for pegmatites represent a quantum shift from the widely held view that the large crystals found in pegmatites are the result of very slow rates of cooling and crystal growth. Experimental and field evidence both suggest that undercooling and disequilibrium crystallization dominate pegmatite crys- tallization. London’s constitutional zone refining model of pegmatite evolution involves disequilibrium crystallization from an undercooled, flux-bearing granitic melt. The melt is not necessarily flux–rich and the model does not require the presence of an aqueous vapor phase. -

Lithium Mineral Evolution and Ecology: Comparison with Boron and Beryllium
Eur. J. Mineral. – 2019, 31, 755 774 To Christian Chopin, Published online 6 June 2019 for 30 years of dedicated service to EJM Lithium mineral evolution and ecology: comparison with boron and beryllium Edward S. GREW1,*, Grete HYSTAD2, Myriam P. C. TOAPANTA2, Ahmed ELEISH3, Alexandra OSTROVERKHOVA4, Joshua GOLDEN5 and Robert M. HAZEN6 1School of Earth and Climate Sciences, 5790 Bryand Global Sciences Center, University of Maine, Orono, ME 04469-5790, USA *Corresponding author, e-mail: [email protected] 2Department of Mathematics, Statistics, and Computer Science, Purdue University Northwest, Hammond, IN 46323, USA 3Tetherless World Constellation, Rensselaer Polytechnic Institute, 110 8th Street, Troy, NY 12180, USA 4Department of Geology, Southern Illinois University, Parkinson, Mail Code 4324, Carbondale, IL 62901, USA 5Department of Geosciences, University of Arizona, Tucson, AZ 85721-0077, USA 6Geophysical Laboratory, Carnegie Institution for Science, 5251 Broad Branch Road NW, Washington, DC 20015, USA Abstract: The idea that the mineralogical diversity now found at or near Earth’s surface was not present for much of the Earth’s history is the essence of mineral evolution, and the geological histories of the 118 Li, 120 Be, and 296 B minerals are not exceptions. Present crustal concentrations are generally too low for Li, Be, and B minerals to form (except tourmaline); this requires further enrichment by 1–2 orders of magnitude by processes such as partial melting and mobilization of fluids. As a result, minerals containing essential Li and Be are first reported in the geologic record at 3.0–3.1 Ga, later than Li-free tourmaline at 3.6 Ga. -

THE 6Th INTERNATIONAL SYMPOSIUM on GRANITIC PEGMATITES
CONTRIBUTIONS TO THE 6th INTERNATIONAL SYMPOSIUM ON GRANITIC PEGMATITES EDITORS WILLIAM B. SIMMONS KAREN L. WEBBER ALEXANDER U. FALSTER ENCARNACIÓN RODA-ROBLES SARAH L. HANSON MARÍA FLORENCIA MÁRQUEZ-ZAVALÍA MIGUEL ÁNGEL Galliski GUEST EDITORS ANDREW P. BOUDREAUX KIMBERLY T. CLARK MYLES M. FELCH KAREN L. MARCHAL LEAH R. GRASSI JON GUIDRY SUSANNA T. KREINIK C. MARK JOHNSON COVER DESIGN BY RAYMOND A. SPRAGUE PRINTED BY RUBELLITE PRESS, NEW ORLEANS, LA PEG 2013: The 6th International Symposium on Granitic Pegmatites PREFACE Phosphate Theme Session Dedicated to: François Fontan, André-Mathieu Fransolet and Paul Keller, It is a pleasure for the organizing committee to phosphates during the evolution of the pegmatites introduce the special session on phosphates, as an and on their relationship to other mineral phases, important part of the program of the 6th such as silicates. These kinds of studies are getting International Symposium on Granitic Pegmatites. more and more abundant, with some interesting The complexity of phosphate associations, examples in this volume. commonly occurring as a mixture of several fine- We take this opportunity to honor François grained phases, make their study difficult. However Fontan, Paul Keller and André-Mathieu Fransolet. over the last decades, the number of publications on These three exceptional researchers have pegmatite phosphate minerals has increased contributed enormously to the advancement in the exponentially as a result of new techniques. The knowledge on phosphates during the last decades. early investigations focused on description, They worked individually and jointly, always in an composition and paragenesis. Now that most of the enthusiastic, effective and tireless way. They passed phases have been extensively described and their knowledge and interest in phosphates onto replacement sequences of secondary phosphates are many younger researchers. -

Anderson & Steinssen
Anderson & Steinssen, Inc. Crystalline Products Company Overview Anderson & Steinssen, Inc. (Abbreviation: ANS) is one of the most reputable and largest laser crystal manufacturers in the world. Our main optical crystal products include the YAG series, which we supply to our cooperative research centers and universities. We have also successfully expanded to manufacturing NLO crystal, BIBO, LBO, KTP, etc, which are widely used in industrial, military, medical, cosmetic and other fields. ANS has a long-term commitment to improvement of and research in growth technology. At ANS, we have crystal growth technology including induction heating and resistance heating, which can meet different customers’ requirements. We are now serving customers throughout America, Korea, Canada, Japan and Germany. Crystal Products Product Name Formula Yttrium aluminium garnet YAG Neodymium-doped yttrium aluminium garnet Nd:YAG Ytterbium-doped yttrium aluminium garnet Yb:YAG Neodymium-cerium double-doped yttrium aluminium garnet (Nd,Ce):YAG Chromium (IV)-doped yttrium aluminium garnet Cr4:YAG Erbium-doped yttrium aluminium garnet Er:YAG Holmium-doped yttrium aluminium garnet Ho:YAG Samarium-doped yttrium aluminium garnet Sm:YAG Dysprosium-doped yttrium aluminium garnet Dy:YAG Holmium-chromium-thulium triple-doped yttrium aluminium Ho:Cr:Tm:YAG garnet Terbium-doped yttrium aluminium garnet Tb:YAG Thulium-doped yttrium aluminium garnet Tm:YAG Cerium(III)-doped yttrium aluminium garnet Ce:YAG Neodymium-doped yttrium lithium fluoride Nd:YLF Calcium gadolinium aluminum -

Re-Assessing the European Lithium Resource Potential
Re-assessing the European lithium resource potential – A review of hard-rock resources and metallogeny Blandine Gourcerol, Eric Gloaguen, Jérémie Melleton, Johann Tuduri, Xavier Galiègue To cite this version: Blandine Gourcerol, Eric Gloaguen, Jérémie Melleton, Johann Tuduri, Xavier Galiègue. Re-assessing the European lithium resource potential – A review of hard-rock resources and metallogeny. Ore Geology Reviews, Elsevier, 2019, 109, pp.494-519. 10.1016/j.oregeorev.2019.04.015. insu-02115174 HAL Id: insu-02115174 https://hal-insu.archives-ouvertes.fr/insu-02115174 Submitted on 30 Apr 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Accepted Manuscript Re-assessing the European lithium resource potential – A review of hard-rock resources and metallogeny B. Gourcerol, E. Gloaguen, J. Melleton, J. Tuduri, Xavier Galiegue PII: S0169-1368(18)30801-1 DOI: https://doi.org/10.1016/j.oregeorev.2019.04.015 Reference: OREGEO 2903 To appear in: Ore Geology Reviews Received Date: 20 September 2018 Revised Date: 15 April 2019 Accepted Date: 23 April 2019 Please cite this article as: B. Gourcerol, E. Gloaguen, J. Melleton, J. Tuduri, X. Galiegue, Re-assessing the European lithium resource potential – A review of hard-rock resources and metallogeny, Ore Geology Reviews (2019), doi: https://doi.org/10.1016/j.oregeorev.2019.04.015 This is a PDF file of an unedited manuscript that has been accepted for publication.