Suplementary Materials. Comparative Interrelationship of the Structural, Nonlinear- Optical and Other Acentric Properties for Oxide, Borate and Carbonate Crystals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Standard X-Ray Diffraction Powder Patterns
NBS MONOGRAPH 25 — SECTION 1 Standard X-ray Diffraction U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS Functions and Activities The functions of the National Bureau of Standards are set forth in the Act of Congress, March 3, 1901, as amended by Congress in Public Law 619, 1950. These include the development and maintenance of the national standards of measurement and the provision of means and methods for making measurements consistent with these standards; the determination of physical constants and properties of materials; the development of methods and instruments for testing materials, devices, and structures; advisory services to government agencies on scien- tific and technical problems; invention and development of devices to serve special needs of the Government; and the development of standard practices, codes, and specifications. The work includes basic and applied research, development, engineering, instrumentation, testing, evaluation, calibration services, and various consultation and information services. Research projects are also performed for other government agencies when the work relates to and supplements the basic program of the Bureau or when the Bureau's unique competence is required. The scope of activities is suggested by the listing of divisions and sections on the inside of the back cover. Publications The results of the Bureau's research are published either in the Bureau's own series of publications or in the journals of professional and scientific societies. The Bureau itself publishes three periodicals available from the Government Printing Office: The Journal of Research, published in four separate sections, presents complete scientific and technical papers; the Technical News Bulletin presents summary and preliminary reports on work in progress; and Basic Radio Propagation Predictions provides data for determining the best frequencies to use for radio communications throughout the world. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -
Evaluation of Aqueous Systems
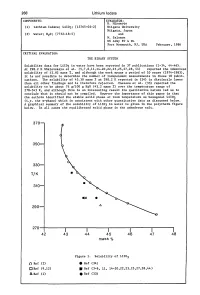
268 Lithium Iodate COMPONENTS: EVALUATOR: H. Miyamoto (1) Lithium Iodate; LiI03; [13765-03-2] Niigata University Niigata, Japan (2) Water; H20; [7732-18-5] and M. Salomon US Army ET & DL Fort Monmouth, NJ, USA February, 1986 CRITICAL EVALUATION: THE BINARY SYSTEM Solubility data for LiI03 in water have been reported in 37 publications (1-34, 44-46). At 298.2 K Shklovskaya et al. (5,7,8,11,14-20,22,23,25,27,28,44) reported the identical solubility of 43.82 mass %, and although the work spans a period of 10 years (1974-1983), it is not possible to determine the number of independent measurements in these 18 publi cations. The solubility of 43.30 mass % at 298.2 K reported in (24) is distinctly lower than all other findings and is therefore rejected. Unezawa et al. (33) reported the solubility to be about 76 g/lOO g H20 (43.2 mass %) over the temperature range of 278-343 K, and although this is an interesting result its qualitative nature led us to conclude that it should not be compiled. However the importance of this paper is that the authors identified the stable solid phase at room temperature as hexagonal LiI03 (i.e. the a-phase) which is consistent with other quantitative data as discussed below. A graphical summary of the solubility of LiI03 in water is given in the polytherm figure below. In all cases the equilibrated solid phase is the anhydrous salt. 370 350 330 T/K 310 290 270-I------.r-----.-----..----......----------. 42 43 44 45 46 47 48 mass % Figure 1. -

Handbook on the Physics and Chemistry of Rare Earths Volume 9 Elsevier, 1987
Handbook on the Physics and Chemistry of Rare Earths volume 9 Elsevier, 1987 Edited by: Karl A. Gschneidner, Jr. and LeRoy Eyring ISBN: 978-0-444-87045-2 by kmno4 Handbook on the Physics and Chemistry of Rare Earths, edited by K.A. Gschneidner, Jr. and L. Eyring © Elsevier Science Publishers B.V., 1987 PREFACE Karl A. GSCHNEIDNER, Jr., and LeRoy EYRING o These elements perplex us in our rearches [sic], baffle us in our speculations, and haunt us in our very dreams. They stretch like an unknown sea before us- mocking, mystifying, and murmuring strange revelations and possibilities. Sir William Crookes (February 16, 1887) There are those who feel that the rare earth elements are destined to play an even greater role in our "high-tech" society in the future than they have in the past. This judgement is based upon the trend of increasing applications resulting from the electronic structures of these materials that lead to their unusual optical, magnetic, electrical and chemical properties so adaptable to the demands now being placed on materials. The "Handbook" seeks to provide topical reviews and compilations of critically reviewed data to aid in the design and fabrication of the required new materials. Four additional chapters in this tradition are the contents of this volume. The development of lasers containing rare earth species sparked an interest in glasses containing these elements. Reisfeld and J0rgensen discuss excited-state phenomena in vitreous rare-earth-containing substances. The description of the chemistry and crystal chemistry of complex inorganic compounds of the rare earths is continued by Niinist6 and Leskelfi from the previous volume. -

Report on the Sonora Lithium Project
Report on the Sonora Lithium Project (Pursuant to National Instrument 43-101 of the Canadian Securities Administrators) Huasabas - Bacadehuachi Area (Map Sheet H1209) Sonora, Mexico o o centered at: 29 46’29”N, 109 6’14”W For 330 – 808 – 4th Avenue S. W. Calgary, Alberta Canada T2P 3E8 Tel. +1-403-237-6122 By Carl G. Verley, P. Geo. Martin F. Vidal, Lic. Geo. Geological Consultant Vice-President, Exploration Amerlin Exploration Services Ltd. Bacanora Minerals Ltd. 2150 – 1851 Savage Road Calle Uno # 312 Richmond, B.C. Canada V6V 1R1 Hermosillo, Mexico Tel. +1-604-821-1088 Tel. +52-662-210-0767 Ellen MacNeill, P.Geo. Geological Consultant RR5, Site25, C16 Prince Albert, SK Canada S6V 5R3 Tel. +1-306-922-6886 Dated: September 5, 2012. Bacanora Minerals Ltd. Report on the Sonora Lithium Project, Sonora, Mexico Date and Signature Page Date Dated Effective: September 5, 2012. Signatures Carl G. Verley, P.Geo. ____________________ Martin F. Vidal, Lic. Geo. Ellen MacNeill, P.Geo. Amerlin Exploration Services Ltd. - Consulting mineral exploration geologists ii Bacanora Minerals Ltd. Report on the Sonora Lithium Project, Sonora, Mexico Table of Contents 1.0 Summary ................................................................................................................................... 1 2.0 Introduction ............................................................................................................................... 3 3.0 Reliance on Other Experts ....................................................................................................... -

Mesoscopic Rydberg-Blockaded Ensembles in the Superatom Regime and Beyond
LETTERS PUBLISHED ONLINE: 12 JANUARY 2015 | DOI: 10.1038/NPHYS3214 Mesoscopic Rydberg-blockaded ensembles in the superatom regime and beyond T. M. Weber, M. Höning, T. Niederprüm, T. Manthey, O. Thomas, V. Guarrera†, M. Fleischhauer, G. Barontini† and H. Ott* The control of strongly interacting many-body systems of a Bose–Einstein condensate of 87Rb atoms. We first load the enables the creation of tailored quantum matter with complex condensate into a one-dimensional optical lattice with a spacing of properties. Atomic ensembles that are optically driven to a 532 nm, to suppress the axial movement of the atoms. We subse- Rydberg state provide many examples for this: atom–atom quently compress the atomic sample in the radial direction to reduce entanglement1,2, many-body Rabi oscillations3, strong its size below the blockade radius and empty all but three (or more) photon–photon interaction4 and spatial pair correlations5. lattice sites using a focused electron beam8–10 (Fig. 1a and Methods). In its most basic form Rydberg quantum matter consists of The atom number within the ensemble (N) can be adjusted between an isolated ensemble of strongly interacting atoms spatially 100 and 500 at a temperature of T D .3.5 0.5/ µK and the typical confined to the blockade volume—a superatom. Here we size of the sample is ≤3 µm in diameter (Fig. 1b). Our preparation demonstrate the controlled creation and characterization scheme is readily scalable to arrays of superatoms (Fig. 1d). of an isolated mesoscopic superatom by means of accurate After preparation we excite the atomic ensemble with a density engineering and excitation to Rydberg p-states. -

Fabrication and Characterization of Rare-Earth Silicide Thin Films
UNIVERSITÉ CATHOLIQUE DE LOUVAIN Ecole Polytechnique de Louvain ICTEAM Fabrication and characterization of rare-earth silicide thin films Dissertation présentée en vue de l’obtention du grade de Docteur en Sciences de l’Ingénieur par Nicolas Reckinger Promoteurs: Prof. Vincent Bayot Prof. Jean-Pierre Raskin Février 2011 UNIVERSITÉ CATHOLIQUE DE LOUVAIN Ecole Polytechnique de Louvain ICTEAM Fabrication and characterization of rare-earth silicide thin films Dissertation présentée en vue de l’obtention du grade de Docteur en Sciences de l’Ingénieur par Nicolas Reckinger Membres du jury: Prof. Vincent Bayot, promoteur Prof. Jean-Pierre Raskin, promoteur Prof. Emmanuel Dubois Prof. Denis Flandre Dr. Thierry Baron Prof. Siegfried Mantl Dr. Xiaohui Tang Prof. Danielle Vanhoenacker-Janvier, président Février 2011 Abstract With the continuous reduction of the dimensions of metal-oxide- semiconductor field-effect transistors (MOSFET), issues related to the formation of source and drain contacts by ion implantation appear. A new source and drain architecture based on metallic contacts over silicon was proposed to replace conventional highly doped extensions. To real- ize such a device, the so-called Schottky barrier (SB) MOSFET, requires working with materials presenting low Schottky barrier heights (SBH) to silicon. For n-type MOSFETs, rare-earth silicides, alloys between silicon and a rare-earth metal, are the best candidates. For Schottky barrier MOSFETs to compete with conventional ones in terms of on- and off- currents, the SBH of rare-earth silicides is intrinsically still too high and must accordingly be further reduced. Such a barrier decrease can be achieved with the dopant segregation technique, where a thin dopant layer is interposed between the silicide and silicon. -

Carbon Mineral Ecology: Predicting the Undiscovered Minerals of Carbon
American Mineralogist, Volume 101, pages 889–906, 2016 Carbon mineral ecology: Predicting the undiscovered minerals of carbon ROBERT M. HAZEN1,*, DANIEL R. HUMMER1, GRETHE HYSTAD2, ROBERT T. DOWNS3, AND JOSHUA J. GOLDEN3 1Geophysical Laboratory, Carnegie Institution, 5251 Broad Branch Road NW, Washington, D.C. 20015, U.S.A. 2Department of Mathematics, Computer Science, and Statistics, Purdue University Calumet, Hammond, Indiana 46323, U.S.A. 3Department of Geosciences, University of Arizona, 1040 East 4th Street, Tucson, Arizona 85721-0077, U.S.A. ABSTRACT Studies in mineral ecology exploit mineralogical databases to document diversity-distribution rela- tionships of minerals—relationships that are integral to characterizing “Earth-like” planets. As carbon is the most crucial element to life on Earth, as well as one of the defining constituents of a planet’s near-surface mineralogy, we focus here on the diversity and distribution of carbon-bearing minerals. We applied a Large Number of Rare Events (LNRE) model to the 403 known minerals of carbon, using 82 922 mineral species/locality data tabulated in http://mindat.org (as of 1 January 2015). We find that all carbon-bearing minerals, as well as subsets containing C with O, H, Ca, or Na, conform to LNRE distributions. Our model predicts that at least 548 C minerals exist on Earth today, indicating that at least 145 carbon-bearing mineral species have yet to be discovered. Furthermore, by analyzing subsets of the most common additional elements in carbon-bearing minerals (i.e., 378 C + O species; 282 C + H species; 133 C + Ca species; and 100 C + Na species), we predict that approximately 129 of these missing carbon minerals contain oxygen, 118 contain hydrogen, 52 contain calcium, and more than 60 contain sodium. -

Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: a Review
resources Review Global Lithium Sources—Industrial Use and Future in the Electric Vehicle Industry: A Review Laurence Kavanagh * , Jerome Keohane, Guiomar Garcia Cabellos, Andrew Lloyd and John Cleary EnviroCORE, Department of Science and Health, Institute of Technology Carlow, Kilkenny, Road, Co., R93-V960 Carlow, Ireland; [email protected] (J.K.); [email protected] (G.G.C.); [email protected] (A.L.); [email protected] (J.C.) * Correspondence: [email protected] Received: 28 July 2018; Accepted: 11 September 2018; Published: 17 September 2018 Abstract: Lithium is a key component in green energy storage technologies and is rapidly becoming a metal of crucial importance to the European Union. The different industrial uses of lithium are discussed in this review along with a compilation of the locations of the main geological sources of lithium. An emphasis is placed on lithium’s use in lithium ion batteries and their use in the electric vehicle industry. The electric vehicle market is driving new demand for lithium resources. The expected scale-up in this sector will put pressure on current lithium supplies. The European Union has a burgeoning demand for lithium and is the second largest consumer of lithium resources. Currently, only 1–2% of worldwide lithium is produced in the European Union (Portugal). There are several lithium mineralisations scattered across Europe, the majority of which are currently undergoing mining feasibility studies. The increasing cost of lithium is driving a new global mining boom and should see many of Europe’s mineralisation’s becoming economic. The information given in this paper is a source of contextual information that can be used to support the European Union’s drive towards a low carbon economy and to develop the field of research. -

United States Patent (19) 11 Patent Number: 4,601,755 Mélard Et Al
United States Patent (19) 11 Patent Number: 4,601,755 Mélard et al. 45 Date of Patent: Jul. 22, 1986 (54) CERIUM BASED GLASSPOLISHING (56) References Cited COMPOSITIONS U.S. PATENT DOCUMENTS 75 Inventors: Pierre Mélard, Lagord; Marcel 3,262,766 7/1966 Nonamaker ........... ... 51/309 Peltier, La Rochelle; Francis Tastu, 3,768,989 10/1973 Goetzinger et 51/309 Nieul/sur/Mer, all of France 4,161,463 7/1979 Myers et al... ... SO2/263 73 Assignee: Rhone-Poulene Specialites 4,62,921 7/1979 Litvinov et al. P ... O - 0 - - - - - - - - - - - A as - - 50/9 Chimiques, Courbevoie, France Primary Examiner-Amelia B. Yarbrough Attorney, Agent, or Firm-Burns, Doane, Swecker & 21 Appl. No.: 635,828 Mathis 22 Filed: Jul, 30, 1984 57 ABSTRACT (30) Foreign Application Priority Data Cerium based glass polishing compositions, well adapted for the polishing, e.g., of optical glass, are com Jul. 29, 1983 FR France ................................ 83 2519 prised of (i) at least one crystalline phase of CeO2 type, 51) Int. Cl'................................................ C09G 1/02 and (ii) a crystalline phase which comprises a rare earth 52 U.S. C. ........................................ 106/3; 51/308; pyrosilicate having the formula Ln2-CeSi2O7, 51/309; 156/DIG. 63; 423/263 wherein Ln is at least one lanthanide or yttrium and x is 58 Field of Search ................... 106/3, 288 B, 287.34; a number ranging from zero to less than 2. 51/309, 308; 423/263; 156/DIG. 63; 502/263, 302, 304,303 59 Claims, No Drawings 4,601,755 1. 2 forming insoluble rare earth compounds, with the num CERIUM BASED GLASS POLISHING ber of equivalents of base being greater than or equal to COMPOSITIONS the number of equivalents of cerium and the pH of the reaction medium being higher than 6; BACKGROUND OF THE INVENTION 5 (b) the resultant precipitate is filtered; 1. -
![PDF Card 3-320 [Dow Chemical Co., Midland, 1 .3805 1 006 67.83 Michigan] 1 .3672 6 300 68.58](https://docslib.b-cdn.net/cover/4533/pdf-card-3-320-dow-chemical-co-midland-1-3805-1-006-67-83-michigan-1-3672-6-300-68-58-1214533.webp)
PDF Card 3-320 [Dow Chemical Co., Midland, 1 .3805 1 006 67.83 Michigan] 1 .3672 6 300 68.58
standard X-ray Diffraction Powder Patterns ^v^iSection 10-Data for 84 Substances ^•2. — Howard E. Swanson, Howard F. McMurdie, Marlene C. Morris lliloise H. Evans, and Boris Paretzkin Assisted by Johan H. deGroot and Simon J. Carmel Institute for Materials Research -y.J National Bureau of Standards ' Washington, D.C. 20234 U.S. DEPARTMENT OF COMMERCE, Refer G. Peterson, Secretary NATIONAL BUREAU OF STANDARDS, Lawrence M. Kushner, AcUng Director, Issued November 1972 Library of Congress Catalog Card Number: 53—61386 National Bureau of Standards Monograph 25 Section 10—Data for 84 Substances Nat. Bur. Stand. (U.S.), Monogr. 25— Sec. 10,161 pages (Nov. 1972) CODEN: NBSMA6 For sale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 (Order by SD Catalog No. C13.44: 25/Sec. 10). Price $2.00 CONTENTS Page Page Introduction 1 Zinc manganese oxide (hetaerolite), ZnMn20^ 61 Experimental patterns: Zinc tin oxide, Zn2Sn04 62 Ammonium aluminum sulfate, NH^AKSO^)^ 5 Calculated patterns: Ammonium copper bromide hydrate, (NH^)2CuBr^"2H20 .. 6 Barium bromide, BaBr2 63 Ammonium iodate, NH^IOj 7 Barium iodide, Bal2 66 Ammonium iron sulfate, NH^Fe(S0^)2 8 Boron oxide, B2O3 phase 1 70 Ammonium magnesium aluminum fluoride, NH^IVIgAIFg ... 9 Calcium iron silicate hydroxide, julgoldite, Barium bromide fluoride, BaBrF 10 Ca2Fe3Si30jo(OH,0)2(OH)2 72 Barium chloride fluoride, BaCIF 11 Calcium malate hydrate, CaC4H405-2H20 76 Barium sulfate (barite), BaSO^ (revised). 12 Cesium lithium cobalt cyanide, CsLiCo(CN)g 79 Cadmium -

Winter 2003 Gems & Gemology
Winter 2003 VOLUME 39, NO. 4 EDITORIAL _____________ 267 Tomorrow’s Challenge: CVD Synthetic Diamonds William E. Boyajian FEATURE ARTICLES _____________ 268 Gem-Quality Synthetic Diamonds Grown by a Chemical Vapor Deposition (CVD) Method Wuyi Wang, Thomas Moses, Robert C. Linares, James E. Shigley, Matthew Hall, and James E. Butler pg. 269 Description and identifying characteristics of Apollo Diamond Inc.’s facetable, single-crystal type IIa CVD-grown synthetic diamonds. 284 Pezzottaite from Ambatovita, Madagascar: A New Gem Mineral Brendan M. Laurs, William B. (Skip) Simmons, George R. Rossman, Elizabeth P. Quinn, Shane F. McClure, Adolf Peretti, Thomas Armbruster, Frank C. Hawthorne, Alexander U. Falster, Detlef Günther, Mark A. Cooper, and Bernard Grobéty A look at the history, geology, composition, and properties of this new cesium-rich member of the beryl group. 302 Red Beryl from Utah: A Review and Update James E. Shigley, Timothy J. Thompson, and Jeffrey D. Keith A report on the geology, history, and current status of the world’s only known occurrence of gem-quality red beryl. pg. 299 REGULAR FEATURES _____________________ 314 Lab Notes • Chrysocolla “owl” agate • Red coral • Coated diamonds • Natural emerald with nail-head spicules • Emerald with strong dichroism • High-R.I. glass imitation of tanzanite • Large clam “pearl” • Blue sapphires with unusual color zoning • Spinel with filled cavities 322 Gem News International • Comparison of three historic blue diamonds • Natural yellow diamond with nickel-related optical centers