EUROPEAN COMMISSION Selection of Chemical Substances to Be
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
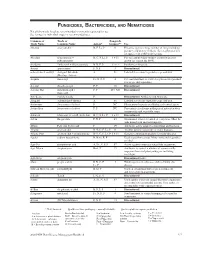
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Expansive and Diverse Phenotypic Landscape of Field Aedes Aegypti Larvae with Differential Susceptibility
bioRxiv preprint doi: https://doi.org/10.1101/2021.06.07.447310; this version posted June 7, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Title Page 2 Full Title: Expansive and diverse phenotypic landscape of field Aedes aegypti larvae with 3 differential susceptibility to temephos: beyond metabolic detoxification 4 Short Title: Gene expression in temephos resistant field populations of Aedes aegypti 5 Authors: Jasmine Morgan1, J. Enrique Salcedo-Sora2*, Omar Triana-Chavez3, Clare Strode1* 6 Author affiliations: 1Department of Biology, Edge Hill University, UK 7 2Institute of Systems, Molecular and Integrative Biology, University of Liverpool, UK 8 3Instituto de Biología, Facultad de Ciencias Exactas y Naturales (FCEN), University of 9 Antioquia, Medellín, Colombia 10 Corresponding authors: * [email protected] (CS) and J.Salcedo- 11 [email protected] (JES-S) 1 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.06.07.447310; this version posted June 7, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 12 Abstract 13 Arboviruses including dengue, Zika and chikungunya are amongst the most significant public 14 health concerns worldwide and their control relies heavily on the use of insecticides to 15 control the vector mosquito Aedes aegypti. -

Transfluthrin (Insecticides, Acaricides and Products to Control Other Arthropods)
Regulation (EU) n°528/2012 concerning the making available on the market and use of biocidal products Evaluation of active substances Assessment Report Transfluthrin (insecticides, acaricides and products to control other arthropods) 13 March 2014 RMS: the Netherlands Transfluthrin (PT18) Assessment report Finalised in the Standing Committee on Biocidal Products at its meeting on 13 March 2014 CONTENTS 1. STATEMENT OF SUBJECT MATTER AND PURPOSE .................................. 4 1.1. Principle of evaluation .................................................................................... 4 1.2. Purpose of the assessment report ................................................................... 4 1.3. Procedure followed .......................................................................................... 4 2. OVERALL SUMMARY AND CONCLUSIONS ................................................... 6 2.1. Presentation of the Active Substance ............................................................. 6 2.1.1. Identity, Physico-Chemical Properties & Methods of Analysis ....... 6 2.1.2. Intended Uses and Efficacy ................................................................ 8 2.1.3. Classification and Labelling .............................................................. 8 2.2. Summary of the Risk Assessment ................................................................ 11 2.2.1. Human Health Risk Assessment ...................................................... 11 2.2.1.1. Hazard identification ........................................................................ -

Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-Target Organisms Katherine Horak U.S
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USDA National Wildlife Research Center - Staff U.S. Department of Agriculture: Animal and Plant Publications Health Inspection Service 2018 Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-target Organisms Katherine Horak U.S. Department of Agriculture, [email protected] Penny M. Fisher Landcare Research Brian M. Hopkins Landcare Research Follow this and additional works at: https://digitalcommons.unl.edu/icwdm_usdanwrc Part of the Life Sciences Commons Horak, Katherine; Fisher, Penny M.; and Hopkins, Brian M., "Pharmacokinetics of Anticoagulant Rodenticides in Target and Non- target Organisms" (2018). USDA National Wildlife Research Center - Staff Publications. 2091. https://digitalcommons.unl.edu/icwdm_usdanwrc/2091 This Article is brought to you for free and open access by the U.S. Department of Agriculture: Animal and Plant Health Inspection Service at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USDA National Wildlife Research Center - Staff ubP lications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Chapter 4 Pharmacokinetics of Anticoagulant Rodenticides in Target and Non-target Organisms Katherine E. Horak, Penny M. Fisher, and Brian Hopkins 1 Introduction The concentration of a compound at the site of action is a determinant of its toxicity. This principle is affected by a variety of factors including the chemical properties of the compound (pKa, lipophilicity, molecular size), receptor binding affinity, route of exposure, and physiological properties of the organism. Many compounds have to undergo chemical changes, biotransformation, into more toxic or less toxic forms. Because of all of these variables, predicting toxic effects and performing risk assess- ments of compounds based solely on dose are less accurate than those that include data on absorption, distribution, metabolism (biotransformation), and excretion of the compound. -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

Insecticide Recommendations for Arkansas Peanuts
PEAnut InSECt COntROl Restricted Minimum days Entry From last Interval Application Insect Insecticide Formulation/Acre lb ai/Acre Acres/Gallon Application/Comments (hours) to Harvest thrips acephate 0.375-0.75 DO NOT graze or feed vines treated with 24 14 Orthene 97 6-12 oz Orthene. (of digging) Treat with foliar insecticides when gamma-cyhalothrin (R) 0.01-0.015 24 14 25% of newly emerged Prolex/Declare 1.25 CS 1.02-1.54 oz 83-125 leaflets show damage imidacloprid In-furrow application/ 12 14 from thrips. Admire Pro 4.6F 7-10.5 fl oz lambda-cyhalothrin (R) 0.02-0.03 DO NOT graze or feed vines treated with 24 14 Warrior II 2.08 CS 1.28-1.92 oz 66.6-100 Warrior. (See Generic Insecticides) phorate (R) DO NOT apply more than 7.5 lb/acre. DO 48 90 Thimet 20 G 5.5 oz/1,000 ft of row NOT graze or feed treated hay or forage to livestock. threecornered beta-cyfluthrin (R) 0.014-0.019 12 14 Alfalfa Hopper Baythroid XL 1 EC 1.8-2.4 oz 53.3-71 bifenthrin (R) 0.033-0.1 DO NOT graze or feed treated hay or forage 12 14 Brigade 2 EC 2.1-6.4 oz 20-60 to livestock. (See Generic Insecticides) carbaryl 1 DO NOT apply during bloom. 12 14 Sevin XLR or 4 F 1 qt 4 Sevin 80 S 1.25 lb esfenvalerate (R) 0.015-0.03 DO NOT graze or feed treated hay or forage 12 21 Asana XL 0.66 EC 2.9-5.8 oz 22-44 to livestock. -

Polystyrene Microplastics Do Not Affect Juvenile Brown Trout (Salmo Trutta F
Schmieg et al. Environ Sci Eur (2020) 32:49 https://doi.org/10.1186/s12302-020-00327-4 RESEARCH Open Access Polystyrene microplastics do not afect juvenile brown trout (Salmo trutta f. fario) or modulate efects of the pesticide methiocarb Hannah Schmieg1*, Sven Huppertsberg2, Thomas P. Knepper2, Stefanie Krais1, Katharina Reitter1, Felizitas Rezbach1, Aki S. Ruhl3,4, Heinz‑R. Köhler1 and Rita Triebskorn1,5 Abstract Background: There has been a rising interest within the scientifc community and the public about the environmen‑ tal risk related to the abundance of microplastics in aquatic environments. Up to now, however, scientifc knowledge in this context has been scarce and insufcient for a reliable risk assessment. To remedy this scarcity of data, we inves‑ tigated possible adverse efects of polystyrene particles (10 4 particles/L) and the pesticide methiocarb (1 mg/L) in juvenile brown trout (Salmo trutta f. fario) both by themselves as well as in combination after a 96 h laboratory expo‑ sure. PS beads (density 1.05 g/mL) were cryogenically milled and fractionated resulting in irregular‑shaped particles (< 50 µm). Besides body weight of the animals, biomarkers for proteotoxicity (stress protein family Hsp70), oxidative stress (superoxide dismutase, lipid peroxidation), and neurotoxicity (acetylcholinesterase, carboxylesterases) were analyzed. As an indicator of overall health, histopathological efects were studied in liver and gills of exposed fsh. Results: Polystyrene particles by themselves did not infuence any of the investigated biomarkers. In contrast, the exposure to methiocarb led to a signifcant reduction of the activity of acetylcholinesterase and the two carboxy‑ lesterases. Moreover, the tissue integrity of liver and gills was impaired by the pesticide. -

Bayesian Nonparametric Model for Clustering Individual Co-Exposure to Pesticides Found in the French Diet
Bayesian nonparametric model for clustering individual co-exposure to pesticides found in the French diet. Amélie Crépet, Jessica Tressou To cite this version: Amélie Crépet, Jessica Tressou. Bayesian nonparametric model for clustering individual co-exposure to pesticides found in the French diet.. 2009. hal-00438796v2 HAL Id: hal-00438796 https://hal.archives-ouvertes.fr/hal-00438796v2 Preprint submitted on 12 Jan 2011 (v2), last revised 4 Feb 2011 (v3) HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Bayesian nonparametric model for clustering individual co-exposure to pesticides found in the French diet. Am´elieCr´epet a & Jessica Tressoub January 12, 2011 aANSES, French Agency for Food, Environmental and Occupational Health Safety, 27-31 Av. G´en´eralLeclerc, 94701 Maisons-Alfort, France bINRA-Met@risk, Food Risk Analysis Methodologies, National Institute for Agronomic Re- search, 16 rue Claude Bernard, 75231 Paris, France Keywords Dirichlet process; Bayesian nonparametric modeling; multivariate Normal mixtures; clustering; multivariate exposure; food risk analysis. Abstract This work introduces a specific application of Bayesian nonparametric statistics to the food risk analysis framework. The goal was to determine the cocktails of pesticide residues to which the French population is simultaneously exposed through its current diet in order to study their possible combined effects on health through toxicological experiments. -

Tenth Meeting of the WHO Vector Control Advisory Group
MEETING REPORT 13–15 May 2019 Tenth meeting of the WHO Vector Control Advisory Group MEETING REPORT 13–15 May 2019 Tenth meeting of the WHO Vector Control Advisory Group WHO/CDS/VCAG/2019.02 © World Health Organization 2019 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial- ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. Tenth meeting of the WHO Vector Control Advisory Group. Geneva: World Health Organization; 2018 (WHO/CDS/VCAG/2019.02). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

The Evaluation of Alternative Toxins to Sodium Monofluoroacetate (1080) for Possum Control
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UNL | Libraries University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Proceedings of the Fifteenth Vertebrate Pest Vertebrate Pest Conference Proceedings Conference 1992 collection March 1992 THE EVALUATION OF ALTERNATIVE TOXINS TO SODIUM MONOFLUOROACETATE (1080) FOR POSSUM CONTROL Charles T. Eason Forest Research Institute, P.O. Box 31-011, Christchurch, New Zealand Follow this and additional works at: https://digitalcommons.unl.edu/vpc15 Part of the Environmental Health and Protection Commons Eason, Charles T., "THE EVALUATION OF ALTERNATIVE TOXINS TO SODIUM MONOFLUOROACETATE (1080) FOR POSSUM CONTROL" (1992). Proceedings of the Fifteenth Vertebrate Pest Conference 1992. 24. https://digitalcommons.unl.edu/vpc15/24 This Article is brought to you for free and open access by the Vertebrate Pest Conference Proceedings collection at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Proceedings of the Fifteenth Vertebrate Pest Conference 1992 by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. THE EVALUATION OF ALTERNATIVE TOXINS TO SODIUM MONOFLUOROACETATE (1080) FOR POSSUM CONTROL CHARLES T. EASON, Forest Research Institute, P.O. Box 31-011, Christchurch, New Zealand ABSTRACT: Possum control in New Zealand is dependent on the use of sodium monofluroacetate (1080) and cyanide. Although 1080 is highly effective, its use is restricted to government staff. Cyanide is available for a wider group of licensed operators, but cyanide "shyness" reduces its effectiveness. An acute toxicity programme has been set up to identify non- anticoagulant toxins that could be used safely by farmers. Dose-ranging studies showed that possums are susceptible to cholecalciferol, calciferol, gliftor, alpha-chloralose, and nicotine, but not to bromethalin. -

RRAC Guidelines on Anticoagulant Rodenticide Resistance Management Editor: Rodenticide Resistance Action Committee (RRAC) of Croplife International Aim
RRAC guidelines on Anticoagulant Rodenticide Resistance Management Editor: Rodenticide Resistance Action Committee (RRAC) of CropLife International Aim This document provides guidance to advisors, national authorities, professionals, practitioners and others on the nature of anticoagulant resistance in rodents, the identification of anticoagulant resistance, strategies for rodenticide application that will avoid the development of resistance and the management of resistance where it occurs. The Rodenticide Resistance Action Committee (RRAC) is a working group within the framework of CropLife International. Participating companies include: Bayer CropScience, BASF, LiphaTech S. A., PelGar, Rentokil Initial, Syngenta and Zapi. Senior technical specialists, with specific expertise in rodenticides, represent their companies on this committee. The RRAC is grateful to the following co-authors: Stefan Endepols, Alan Buckle, Charlie Eason, Hans-Joachim Pelz, Adrian Meyer, Philippe Berny, Kristof Baert and Colin Prescott. Photos provided by Stefan Endepols. Contents 1. Introduction ............................................................................................................................................................................................................. 2 2. Classification and history of rodenticide compounds ..............................................................................................3 3. Mode of action of anticoagulant rodenticides, resistance mechanisms, and resistance mutations ......................................................................................................6