Denatured Alcohol As a Frequent Source of Methanol Intoxication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SAFETY DATA SHEET Isopropyl Alcohol
SAFETY DATA SHEET Isopropyl Alcohol Section 1. Identification GHS product identifier : Isopropyl Alcohol Chemical name : Isopropyl alcohol Other means of : isopropanol; 2-Propanol identification Product type : Liquid. Product use : Synthetic/Analytical chemistry. Synonym : isopropanol; 2-Propanol SDS # : 001105 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE LIQUIDS - Category 2 substance or mixture EYE IRRITATION - Category 2A SPECIFIC TARGET ORGAN TOXICITY (SINGLE EXPOSURE) (Narcotic effects) - Category 3 GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : May form explosive mixtures with air. Highly flammable liquid and vapor. Causes serious eye irritation. May cause drowsiness or dizziness. Precautionary statements General : Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Prevention : Wear protective gloves. Wear eye or face protection. Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Use explosion- proof electrical, ventilating, lighting and all material-handling equipment. Use only non- sparking tools. Take precautionary measures against static discharge. Keep container tightly closed. Use only outdoors or in a well-ventilated area. Avoid breathing vapor. Wash hands thoroughly after handling. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. -

N-BUTYL ALCOHOL
Right to Know Hazardous Substance Fact Sheet Common Name: n-BUTYL ALCOHOL Synonyms: Propyl Carbinol; n-Butanol CAS Number: 71-36-3 Chemical Name: 1-Butanol RTK Substance Number: 1330 Date: November 1998 Revision: January 2008 DOT Number: UN 1120 Description and Use EMERGENCY RESPONDERS >>>> SEE BACK PAGE n-Butyl Alcohol is a colorless liquid with a strong, sweet Hazard Summary alcohol odor. It is used as a solvent for fats, waxes, shellacs, Hazard Rating NJDOH NFPA resins, gums, and varnish, in making hydraulic fluids, and in HEALTH - 2 medications for animals. FLAMMABILITY - 3 REACTIVITY - 0 f ODOR THRESHOLD = 1 to 15 ppm FLAMMABLE f Odor thresholds vary greatly. Do not rely on odor alone to POISONOUS GASES ARE PRODUCED IN FIRE determine potentially hazardous exposures. CONTAINERS MAY EXPLODE IN FIRE Hazard Rating Key: 0=minimal; 1=slight; 2=moderate; 3=serious; Reasons for Citation 4=severe f n-Butyl Alcohol is on the Right to Know Hazardous f n-Butyl Alcohol can affect you when inhaled and by Substance List because it is cited by OSHA, ACGIH, DOT, passing through the skin. NIOSH, DEP, IRIS, NFPA and EPA. f Contact can irritate and burn the skin. f This chemical is on the Special Health Hazard Substance f n-Butyl Alcohol can irritate and burn the eyes with possible List. eye damage. f Inhaling n-Butyl Alcohol can irritate the nose, throat and lungs. f Exposure to n-Butyl Alcohol can cause headache, dizziness, nausea and vomiting. SEE GLOSSARY ON PAGE 5. f n-Butyl Alcohol can damage the liver, kidneys, hearing, and sense of balance. -
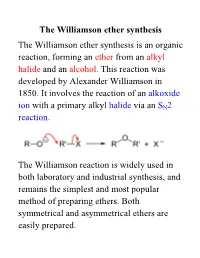
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

Parks Denatured Alcohol
Material Safety Data Sheet Section 1 General Information Manufacturer: Zinsser Company, Inc. 173 Belmont Drive Somerset, NJ 08875 (732) 469-8100 Emergency Telephone: Chemtrec (800) 424-9300 Date: December 1, 2006 Product Name: Parks Denatured Alcohol Codes: 002121 002122 002123 002125 Section 2 Hazardous Ingredients OSHA ACGIH Hazardous Component CAS# PEL TLV Ethanol 64-17-5 1000 ppm 1000 ppm Methanol 67-56-1 200 ppm 200 ppm 250 ppm STEL Methyl Isobutyl Ketone 108-10-1 100 ppm 50 ppm 75 ppm STEL Ethyl Acetate 141-78-6 400 ppm 400 ppm Rubber Solvent 64742-89-8 500 ppm 400 ppm Section 3 Hazard Identification Emergency Overview: This product is a clear liquid with a characteristic smell and flash point of 41oF. Primary Routes of Exposure: Skin Contact Eye Contact Inhalation Potential Acute Health Effects: Eye: Contact may cause eye irritation. N/A: Not Applicable N/D: Not Determined N/E: Not Established N/R: Not Required Est.: Estimated Parks Denatured Alcohol (12-01-06) Page 1 of 6 pages. Skin: May cause skin irritation. Repeated or prolonged contact with skin may cause dermatitis. Ingestion: May be fatal or cause blindness if swallowed. Inhalation: May cause respiratory tract irritation. Potential Chronic Health Effects: Signs and Symptoms: Prolonged exposure to excessive concentrations of ethanol may result in irritation of mucous membranes, headache, drowsiness, fatigue and narcosis. Methanol is also narcotic in effect and its effects are cumulative. Overexposure to methanol can result in acidosis and visual disturbances which may progress to permanent loss of vision. (See also Sections 4, 8, and 11for related information) Section 4 First Aid Measures Eye contact: Immediately flush eyes with water for at least 15 minutes. -

How Is Alcohol Metabolized by the Body?
Overview: How Is Alcohol Metabolized by the Body? Samir Zakhari, Ph.D. Alcohol is eliminated from the body by various metabolic mechanisms. The primary enzymes involved are aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase. Variations in the genes for these enzymes have been found to influence alcohol consumption, alcohol-related tissue damage, and alcohol dependence. The consequences of alcohol metabolism include oxygen deficits (i.e., hypoxia) in the liver; interaction between alcohol metabolism byproducts and other cell components, resulting in the formation of harmful compounds (i.e., adducts); formation of highly reactive oxygen-containing molecules (i.e., reactive oxygen species [ROS]) that can damage other cell components; changes in the ratio of NADH to NAD+ (i.e., the cell’s redox state); tissue damage; fetal damage; impairment of other metabolic processes; cancer; and medication interactions. Several issues related to alcohol metabolism require further research. KEY WORDS: Ethanol-to acetaldehyde metabolism; alcohol dehydrogenase (ADH); aldehyde dehydrogenase (ALDH); acetaldehyde; acetate; cytochrome P450 2E1 (CYP2E1); catalase; reactive oxygen species (ROS); blood alcohol concentration (BAC); liver; stomach; brain; fetal alcohol effects; genetics and heredity; ethnic group; hypoxia The alcohol elimination rate varies state of liver cells. Chronic alcohol con- he effects of alcohol (i.e., ethanol) widely (i.e., three-fold) among individ- sumption and alcohol metabolism are on various tissues depend on its uals and is influenced by factors such as strongly linked to several pathological concentration in the blood T chronic alcohol consumption, diet, age, consequences and tissue damage. (blood alcohol concentration [BAC]) smoking, and time of day (Bennion and Understanding the balance of alcohol’s over time. -

Non-Beverage Alcohol Consumption & Harm Reduction Trends
Non-beverage Alcohol Consumption & Harm Reduction Trends A Report for the Thunder Bay Drug Strategy Prepared by Kim Ongaro HBSW Placement Lakehead University June 15, 2017 Non-beverage Alcohol Consumption & Harm Reduction Trends What is non-beverage alcohol? Non-beverage alcohol can go by many names in the literature. Broadly, it is understood to be liquids containing a form of alcohol that is not intended for human consumption (e.g., mouthwash, hand sanitizer, etc.) that are consumed instead of beverage alcohol for the purposes of intoxication or a “high” (Crabtree, Latham, Bird, & Buxton, 2016; Egbert, Reed, Powell, Liskow, & Liese, 1985). Within the literature, there are different definitions for non- beverage alcohols, including surrogate alcohol, illicit alcohol and unrecorded alcohol. Unrecorded alcohol, as defined by the World Health Organization, is untaxed alcohol outside of government regulation including legal or illegal homemade alcohol, alcohol that is smuggled from an outside country (and therefore is not tracked by its sale within the country of consumption), and alcohol of the “surrogate” nature (World Health Organization Indicator and Measurement Registry, 2011). Surrogate alcohol is alcohol that is not meant for human consumption, and is generally apparent as high concentrations of ethanol in mouthwash, hand sanitizers, and other household products (Lachenmeier, Rehm, & Gmel, 2007; World Health Organization Indicator and Measurement Registry, 2011). Surrogate alcohols also include substances containing methanol, isopropyl alcohol, and ethylene glycol (Lachenmeier et al., 2007). Nonbeverage alcohol and surrogate alcohol can be used interchangeably, but Lachenmeier et al., (2007), goes even further to include alcohol that is homemade in their definition of surrogate alcohol, as they stated that this alcohol is sometimes created using some form of non-beverage alcohol. -

Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to Α
PHOSPHINE-CATALYZED ADDITIONS OF NUCLEOPHILES AND ELECTROPHILES TO α,β–UNSATURATED CARBONYL COMPOUNDS Reported by Michael Scott Bultman November 4, 2004 INTRODUCTION Organophosphorous compounds are becoming increasingly important in organic synthesis. Phosphines serve as precursors to phosphonium ylides in the Wittig reaction,1 and as nucleophilic triggers in the Mitsunobu2 and Staudinger3 reactions. In these processes, the phosphine is stoichiometrically consumed and converted into a phosphine oxide. Phosphines are also commonly used as ligands for transition metal-catalyzed reactions, to modulate reactivity and stereocontrol.4 On the other hand, the use of phosphines as nucleophilic catalysts for organic reactions has only gained attention in the last ten years. First reported by Rauhut and Currier in 1963,5 phosphine catalysis has since been reinvestigated after the phosphine ligands in some transition-metal-catalyzed reactions were found to be better catalysts than the metal/phosphine complexes alone!6 Phosphines are well suited for catalyzing the addition of both nucleophiles and electrophiles to electron deficient alkenes, alkynes, and allenes. Activation of these α,β-unsaturated carbonyl systems with the phosphine enables the formation of new bonds at the α-, β-, and γ-positions. This report will highlight these different modes of addition to α,β-unsaturated carbonyl systems under phosphine catalysis that allow for the formation of a wide array of products from a single class of substrates. GENERAL REACTIVITY OF PHOSPHINES Key characteristics required for successful nucleophilic catalysis lie in the balance of leaving group ability, nucleophilicity, and ease of ylid formation. Increasing leaving group ability can often be + correlated with decreasing basicity. -

Sugar Alcohols—Their Role in the Modern World of Sweeteners: a Review
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Springer - Publisher Connector Eur Food Res Technol (2015) 241:1–14 DOI 10.1007/s00217-015-2437-7 REVIEW PAPER Sugar alcohols—their role in the modern world of sweeteners: a review Małgorzata Grembecka Received: 31 August 2014 / Revised: 16 December 2014 / Accepted: 13 February 2015 / Published online: 28 February 2015 © The Author(s) 2015. This article is published with open access at Springerlink.com Abstract Epidemic obesity and diabetes encouraged the observed. It was mainly due to the developments in bio- changes in population lifestyle and consumers’ food prod- logical studies, the change of a population lifestyle and the ucts awareness. Food industry has responded people’s increase in the consumer awareness concerning food prod- demand by producing a number of energy-reduced prod- ucts. The health quality of food depends mainly on nutri- ucts with sugar alcohols as sweeteners. These compounds ents, but also on foreign substances such as food additives. are usually produced by a catalytic hydrogenation of carbo- The presence of foreign substances in the food can be justi- hydrates, but they can be also found in nature in fruits, veg- fied, allowed or tolerated only when they are harmless to etables or mushrooms as well as in human organism. Due our health. Epidemic obesity and diabetes encouraged the to their properties, sugar alcohols are widely used in food, growth of the artificial sweetener industry. There are more beverage, confectionery and pharmaceutical industries and more people who are trying to lose weight or keeping throughout the world. -

Safety Data Sheet Denatured Alcohol
Safety Data Sheet Denatured Alcohol SECTION I - IDENTIFICATION PRODUCT NAME: Denatured Alcohol PRODUCT CODE: 6210 PRODUCT USE: Industrial Cleaner COMPANY NAME: QuestVapco Corporation COMPANY ADDRESS: PO Box 624 Brenham, TX 77834 COMPANY PHONE: 1-800-231-0454 EMERGENCY PHONE: 800-255-3924 SECTION II – HAZARDS IDENTIFICATION CLASSIFICATION: Flammable Liquid: Category 2 Skin Irritant: Category 2 Eye Irritant: Category 2A HAZARD STATEMENT(S): Danger: Highly flammable liquid and vapor Causes skin and serious eye irritation. This product contains the following percentage of chemicals of unknown toxicity: 0% PRECAUTIONARY STATEMENTS: Keep away from heat, sparks, open flames, and hot surfaces. -No smoking. Keep container tightly closed. Wear protective gloves and eye protection. If on skin (or hair): Take off immediately all contaminated clothing and wash it before reuse. Rinse skin with water or shower. If skin irritation occurs: Get medical advice or attention. If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice or attention. In case of fire use dry chemical, foam, or carbon dioxide. Store in a well- ventilated place. Keep cool. Dispose of contents and container in accordance with local, state, and national regulations. Wash hands thoroughly after handling. For large quantities: Ground or bond container and receiving equipment. Use explosion-proof electrical, ventilating, and lighting equipment. Use only non-sparking tools. Take precautionary measures against static discharge. SYMBOL: HAZARDS NOT OTHERWISE CLASSIFIED: N/A SECTION III – COMPOSITION/INFORMATION ON INGREDIENTS HAZARDOUS INGREDIENT CAS NUMBER PERCENT Ethyl Alcohol 64-17-5 60%-100% Isopropyl Alcohol 67-63-0 3-7% SECTION IV - FIRST AID MEASURES EYES: If in eyes: Rinse cautiously with water for several minutes. -

Core Alcohol and Drug Survey
Form 194 Core Alcohol and Drug Survey For additional use: Long Form A 0 1 2 3 4 5 6 7 8 9 B 0 1 2 3 4 5 6 7 8 9 FIPSE Core Analysis Grantee Group Core Institute C 0 1 2 3 4 5 6 7 8 9 Student Health Programs 0 1 2 3 4 5 6 7 8 9 Southern Illinois University D Please use a number 2 Pencil. Carbondale, IL 62901 E 0 1 2 3 4 5 6 7 8 9 1. Classification: 2. Age: 3. Ethnic origin: 4. Marital status: Freshman . American Indian/ Single . Sophomore . Alaskan Native . Married . Junior . 0 0 Hispanic . Separated . Senior. 1 1 Asian/Pacific Islander . Divorced. Grad/professional . 2 2 White (non-Hispanic) . Widowed . Not seeking a 3 3 Black (non-Hispanic) . degree . 4 4 Other . 7. Are you working? Other . 5 5 Ye s, full-time . 6 6 6. Is your current residence Ye s, part-time . 5. Gender: 7 7 as a student: No . Male . 8 8 On-campus . Female . 9 9 Off-campus . 8. Living arrangements: A. Where: (mark best answer) 9. Approximate cumulative grade point average: (choose one) House/apartment/etc. Residence hall . A+AA- B+BB- C+CC- D+DD-F Approved housing. Fraternity or sorority . 10. Some students have indicated that alcohol or drug use at parties they attend in and Other . around campus reduces their enjoyment, often leads to negative situations, and therefore, they would rather not have alcohol and drugs available and used. Other B. With whom: students have indicated that alcohol and drug use at parties increases their (mark all that apply) enjoyment, often leads to positive situations, and therefore, they would rather have With roommate(s). -

Sugar Alcohols: a Review
International Journal of PharmTech Research CODEN (USA): IJPRIF, ISSN: 0974-4304, ISSN(Online): 2455-9563 Vol.9, No.7, pp 407-413, 2016 Sugar alcohols: A review Ramezan Ali Mahian1, Vahid Hakimzadeh2* 1,2Department of Food Science and Technology, Quchan Branch, Islamic Azad University, Quchan, Iran Abstract : Sugar alcohols are extensively used as sweetening agents. They sometimes possess advantages over the parent sugars in sweetness, caloric reduction and non-cariogenicity. The physical status of carbohydrates in food and confectionery affects both the properties of the product during production and the quality of the final product. Sugar alcohols, such as xylitol, mannitol, sorbitol, and erythritol are emerging food ingredients that provide similar or better sweetness/sensory properties of sucrose, but are less calorigenic. Also, sugar alcohols can be converted into commodity chemicals through chemical catalysis. Biotechnological production offers the safe and sustainable supply of sugar alcohols from renewable biomass. These compounds are usually produced by a catalytic hydrogenation of carbohydrates, but they can be also found in nature in fruits, vegetables or mushrooms as well as in human organism. Due to their properties, sugar alcohols are widely used in food, beverage, confectionery and pharmaceutical industries throughout the world. They have found use as bulk sweeteners that promote dental health and exert prebiotic effect. They are added to foods as alternative sweeteners what might be helpful in the control of calories intake. Consumption of low-calorie foods by the worldwide population has dramatically increased, as well as health concerns associated with the consequent high intake of sweeteners. Key words: Sugar alcohols, low-calorie, Sugar-free products, sweetener. -

Surrogate Alcohol: What Do We Know and Where Do We Go?
Alcoholism: Clinical and Experimental Research Vol. 31, No. 10 October 2007 Surrogate Alcohol: What Do We Know and Where Do We Go? Dirk W. Lachenmeier, Ju¨rgen Rehm, and Gerhard Gmel Background: Consumption of surrogate alcohols (i.e., nonbeverage alcohols and illegally pro- duced alcohols) was shown to impact on different causes of death, not only poisoning or liver dis- ease, and appears to be a major public health problem in Russia and elsewhere. Methods: A computer-assisted literature review on chemical composition and health conse- quences of ‘‘surrogate alcohol’’ was conducted and more than 70 references were identified. A wider definition of the term ‘‘surrogate alcohol’’ was derived, including both nonbeverage alcohols and illegally produced alcohols that contain nonbeverage alcohols. Results: Surrogate alcohol may contain substances that cause severe health consequences including death. Known toxic constituents include lead, which may lead to chronic toxicity, and methanol, which leads to acute poisoning. On the other hand, the role of higher alcohols (e.g., propanol, isobutanol, and isoamyl alcohol) in the etiology of surrogate-associated diseases is cur- rently unclear. Whether other constituents of surrogates have contributed to the high all-cause mortality over and above the effect of ethanol in recent studies also remains unclear. Conclusions: Given the high public health importance associated with the consumption of sur- rogate alcohols, further knowledge on its chemical composition is required as well as research on its links to various disease endpoints should be undertaken with priority. Some interventions to reduce the harm resulting from surrogate alcohol could be undertaken already at this point.