Dissolution Kinetics of Sulfate Minerals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium
Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium Glyn R. Fox Environmental Management Branch Environmental Protection Division September 24, 2007 Victoria, British Columbia Table of Contents Page 1. Introduction ……………………………………………………………………… 4 2. Details Related to Derivation of Proposed 2007 Draft Matrix Soil Standards 2.1 Derivation of Human Health Protection Standards – Intake of contaminated soil ………………………………………………………….. 7 2.2 Derivation of Human health Protection Standards – Groundwater used for drinking water ……………………………….…………….......... 7 2.3 Derivation of Environmental Protection Standards – Toxicity to soil invertebrates and plants ……………………………………….......... 9 2.4 Derivation of Environmental Protection Standards – Livestock ingesting soil and fodder ………………………………………………… 9 2.5 Derivation of Environmental Protection Standards – Major microbial function impairment ………………………………….……… 10 2.6 Derivation of Environmental Protection Standards – Groundwater flow to surface water used by aquatic life ..…………………………… 10 2.7 Derivation of Environmental Protection Standards – Groundwater used for livestock watering ……………………………………………… 11 2.8 Derivation of Environmental Protection Standards – Groundwater used for irrigation ………………………………………………………… 12 2.9 CSST “Background” Adjustment ……………………..……………… 12 2.10 Application of CSST Rounding-off Rule ……………………………… 12 3. References …….…………………………………………………………………… 13 2 Table of Contents (continued) Page 4. Exhibits Exhibit 1. Proposed 2007 Draft Matrix Soil Standards for Barium ……..… 19 5. Tables -

Barite (Barium)
Barite (Barium) Chapter D of Critical Mineral Resources of the United States—Economic and Environmental Geology and Prospects for Future Supply Professional Paper 1802–D U.S. Department of the Interior U.S. Geological Survey Periodic Table of Elements 1A 8A 1 2 hydrogen helium 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 lithium beryllium boron carbon nitrogen oxygen fluorine neon 6.94 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 13 14 15 16 17 18 sodium magnesium aluminum silicon phosphorus sulfur chlorine argon 22.99 24.31 3B 4B 5B 6B 7B 8B 11B 12B 26.98 28.09 30.97 32.06 35.45 39.95 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 potassium calcium scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc gallium germanium arsenic selenium bromine krypton 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.93 58.69 63.55 65.39 69.72 72.64 74.92 78.96 79.90 83.79 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 rubidium strontium yttrium zirconium niobium molybdenum technetium ruthenium rhodium palladium silver cadmium indium tin antimony tellurium iodine xenon 85.47 87.62 88.91 91.22 92.91 95.96 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 cesium barium hafnium tantalum tungsten rhenium osmium iridium platinum gold mercury thallium lead bismuth polonium astatine radon 132.9 137.3 178.5 180.9 183.9 186.2 190.2 192.2 195.1 197.0 200.5 204.4 207.2 209.0 (209) (210)(222) 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 -

Mineralogy of the Martian Surface
EA42CH14-Ehlmann ARI 30 April 2014 7:21 Mineralogy of the Martian Surface Bethany L. Ehlmann1,2 and Christopher S. Edwards1 1Division of Geological & Planetary Sciences, California Institute of Technology, Pasadena, California 91125; email: [email protected], [email protected] 2Jet Propulsion Laboratory, California Institute of Technology, Pasadena, California 91109 Annu. Rev. Earth Planet. Sci. 2014. 42:291–315 Keywords First published online as a Review in Advance on Mars, composition, mineralogy, infrared spectroscopy, igneous processes, February 21, 2014 aqueous alteration The Annual Review of Earth and Planetary Sciences is online at earth.annualreviews.org Abstract This article’s doi: The past fifteen years of orbital infrared spectroscopy and in situ exploration 10.1146/annurev-earth-060313-055024 have led to a new understanding of the composition and history of Mars. Copyright c 2014 by Annual Reviews. Globally, Mars has a basaltic upper crust with regionally variable quanti- by California Institute of Technology on 06/09/14. For personal use only. All rights reserved ties of plagioclase, pyroxene, and olivine associated with distinctive terrains. Enrichments in olivine (>20%) are found around the largest basins and Annu. Rev. Earth Planet. Sci. 2014.42:291-315. Downloaded from www.annualreviews.org within late Noachian–early Hesperian lavas. Alkali volcanics are also locally present, pointing to regional differences in igneous processes. Many ma- terials from ancient Mars bear the mineralogic fingerprints of interaction with water. Clay minerals, found in exposures of Noachian crust across the globe, preserve widespread evidence for early weathering, hydrothermal, and diagenetic aqueous environments. Noachian and Hesperian sediments include paleolake deposits with clays, carbonates, sulfates, and chlorides that are more localized in extent. -
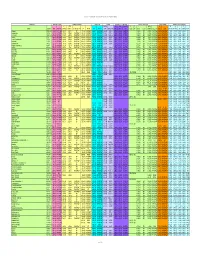
Chemical-Specific Parameters Supporting Table May 2016 Analyte
Regional Screening Level (RSL) Chemical-specific Parameters Supporting Table May 2016 Contaminant Molecular Weight Volatility Parameters Melting Point Density Diffusivity in Air and Water Partition Coefficients Water Solubility Tapwater Dermal Parameters H` (atm- Density Dia Diw Dia and Diw Kd Kd Koc log Kow S B τevent t* Kp 3 3 2 2 Analyte CAS No. MW MW Ref (unitless) m /mole) H` and HLC Ref VP VP Ref MP MP Ref (g/cm ) Density Ref (cm /s) (cm /s) Ref (L/kg) Ref (L/kg) Koc Ref (unitless) log Kow Ref (mg/L) S Ref (unitless) (hr/event) (hr) (cm/hr) K Ref Acephate 30560-19-1 1.8E+02 PHYSPRO 2.0E-11 5.0E-13 EPI 1.7E-06 PHYSPROP 8.8E+01 PHYSPROP 1.4E+00 CRC89 3.7E-02 8.0E-06 WATER9 1.0E+01 EPI -8.5E-01 PHYSPRO 8.2E+05 PHYSPROP 2.1E-04 1.1E+00 2.7E+00 4.0E-05 EPI Acetaldehyde 75-07-0 4.4E+01 PHYSPRO 2.7E-03 6.7E-05 PHYSPROP 9.0E+02 PHYSPROP -1.2E+02 PHYSPROP 7.8E-01 CRC89 1.3E-01 1.4E-05 WATER9 1.0E+00 EPI -3.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.3E-03 1.9E-01 4.5E-01 5.3E-04 EPI Acetochlor 34256-82-1 2.7E+02 PHYSPRO 9.1E-07 2.2E-08 PHYSPROP 2.8E-05 PHYSPROP 1.1E+01 PubChem 1.1E+00 PubChem 2.2E-02 5.6E-06 WATER9 3.0E+02 EPI 3.0E+00 PHYSPRO 2.2E+02 PHYSPROP 3.1E-02 3.4E+00 8.2E+00 5.0E-03 EPI Acetone 67-64-1 5.8E+01 PHYSPRO 1.4E-03 3.5E-05 PHYSPROP 2.3E+02 PHYSPROP -9.5E+01 PHYSPROP 7.8E-01 CRC89 1.1E-01 1.2E-05 WATER9 2.4E+00 EPI -2.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.5E-03 2.2E-01 5.3E-01 5.1E-04 EPI Acetone Cyanohydrin 75-86-5 8.5E+01 PHYSPRO 8.1E-08 2.0E-09 PHYSPROP 3.4E-01 PHYSPROP -1.9E+01 PHYSPROP 9.3E-01 CRC89 8.6E-02 1.0E-05 WATER9 1.0E+00 -

I Identification and Characterization of Martian Acid-Sulfate Hydrothermal
Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation of Instrumentation Techniques and Geochemical Processes Through Laboratory Experiments and Terrestrial Analog Studies by Sarah Rose Black B.A., State University of New York at Buffalo, 2004 M.S., State University of New York at Buffalo, 2006 A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment of the requirement for the degree of Doctor of Philosophy Department of Geological Sciences 2018 i This thesis entitled: Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation of Instrumentation Techniques and Geochemical Processes Through Laboratory Experiments and Terrestrial Analog Studies written by Sarah Rose Black has been approved for the Department of Geological Sciences ______________________________________ Dr. Brian M. Hynek ______________________________________ Dr. Alexis Templeton ______________________________________ Dr. Stephen Mojzsis ______________________________________ Dr. Thomas McCollom ______________________________________ Dr. Raina Gough Date: _________________________ The final copy of this thesis has been examined by the signatories, and we find that both the content and the form meet acceptable presentation standards of scholarly work in the above mentioned discipline. ii Black, Sarah Rose (Ph.D., Geological Sciences) Identification and Characterization of Martian Acid-Sulfate Hydrothermal Alteration: An Investigation -

Efflorescent Iron Sulfate Minerals: Paragenesis, Relative Stability, and Environmental Impact
American Mineralogist, Volume 88, pages 1919–1932, 2003 Efflorescent iron sulfate minerals: Paragenesis, relative stability, and environmental impact JEANETTE K. JERZ* AND J. DONALD RIMSTIDT Department of Geological Sciences, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24061, U.S.A. ABSTRACT This study of a pyrrhotite-dominated massive sulfide deposit in the Blue Ridge province in south- western Virginia shows that sulfate minerals formed by the oxidation of the pyrrhotite transform from one to another by a combination of oxidation, dehydration, and neutralization reactions. Significant quantities of sulfate minerals occur in the underground adits (Area I) and under overhangs along the high sidewall of the adjoining open pit (Area II). Samples from this site were analyzed to determine mineralogy, equilibrium relative humidity, chemical composition, and acid generation potential. In Area I, pyrrhotite oxidizes to marcasite + melanterite, which eventually oxidizes to melanterite + sul- furic acid. Melanterite is extruded from the rocks as a result of the volume change associated with this reaction. It accumulates in piles where halotrichite, copiapite, and fibroferrite form. In Area II, FeSO4 solutions produced by pyrrhotite oxidation migrate to the exposed pit face, where they evaporate to form melanterite. The melanterite rapidly dehydrates to form rozenite, which falls into a pile at the base of the wall, where melanterite, copiapite, and halotrichite are present. The observed paragenesis a a can be understood using a log O2 – log H2O diagram that we developed from published thermody- namic data and observations of coexisting phases. Dissolution experiments showed that fibroferrite-rich samples had the highest acid producing po- tential, followed by copiapite-rich samples and then halotrichite-rich samples. -

Massachusetts Chemical Fact Sheet
Massachusetts Chemical Fact Sheet Hexavalent Chromium Table 1: HEXAVALENT CHROMIUM COMPOUNDS: Compounds SELECTED EXAMPLES* Compound Chemical Formula CAS # This fact sheet is part of a series of chemical fact sheets Ammonium chromate (NH ) Cr0 7788-98-9 developed by TURI to help Massachusetts companies, 4 2 4 community organizations and residents understand the Ammonium dichromate (NH4)2Cr2O7 7789-09-5 chemical’s use and health and environmental effects, as Barium chromate BaCrO4 10294-40-3 well as the availability of safer alternatives. tert-Butyl Chromate [(CH3)3CO]2CrO2 1189-85-1 Hexavalent chromium compounds are a toxic form of Calcium chromate CaCrO4 13765-19-0 chromium and are used in a variety of industrial processes Chromic acid H2CrO4 7738-94-5 and products. Chromium VI chloride CrCl6 14986-48-2 Hexavalent chromium compounds are human carcinogens, Chromic trioxide CrO3 1333-82-0 mutagens and developmental toxicants and are acutely Hexavalent chromium ion Cr6+ 18540-29-9 toxic. Non-hexavalent chromium compounds do not pose Lead chromate PbCrO4 7758-97-6 the same level of concern with regard to either chronic or Lead chromate oxide PbCrO4-PbO 8454-12-1 acute toxicity. Potassium chlorochromate KCrO3Cl 16037-50-6 Until 2011, all chromium compounds were treated as Potassium chromate K2CrO4 7789-00-6 a single category under TURA. Beginning with Potassium dichromate K Cr O 7778-50-9 reporting year 2012, hexavalent chromium 2 2 7 compounds are reportable under TURA as a Silver chromate Ag2CrO4 7784-01-2 separate category and are designated as a Higher Sodium chromate Na2CrO4 7775-11-3 Hazard Substance, which lowers the reporting Sodium dichromate 7789-12-0 threshold to 1,000 lb/year. -

Mineralogical and Geochemical Indicators of Subaerial Weathering in the Pozzolane Rosse Ignimbrite (Alban Hills Volcanic District, Italy)
Georgia State University ScholarWorks @ Georgia State University Geosciences Theses Department of Geosciences 4-27-2010 Mineralogical and Geochemical Indicators of Subaerial Weathering in the Pozzolane Rosse Ignimbrite (Alban Hills Volcanic District, Italy) Jennifer M. Dickie Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/geosciences_theses Part of the Geography Commons, and the Geology Commons Recommended Citation Dickie, Jennifer M., "Mineralogical and Geochemical Indicators of Subaerial Weathering in the Pozzolane Rosse Ignimbrite (Alban Hills Volcanic District, Italy)." Thesis, Georgia State University, 2010. https://scholarworks.gsu.edu/geosciences_theses/23 This Thesis is brought to you for free and open access by the Department of Geosciences at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Geosciences Theses by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. MINERALOGICAL AND GEOCHEMICAL INDICATORS OF SUBAERIAL WEATHERING IN THE POZZOLANE ROSSE IGNIMBRITE (ALBAN HILLS VOLCANIC DISTRICT, ITALY) by JENNIFER M. DICKIE Under the direction of Dr. Daniel Deocampo ABSTRACT The Pozzolane Rosse ignimbrite [PR] (457±4 ka) in the Alban Hills Volcanic District, Rome, Italy was exposed ~ 40 ka prior to a subsequent volcanic event which coverd it entirely. XRF, XRD, and clay separation results from PR samples from INGV and CA1 boreholes and Castel di Leva quarry show evidence of paleopedogenesis. All locations display loss of base cations, loss of K is consistent with XRD datat showing dissolution or alteration of leucite to analcime. Accumulation of Al and high L.O.I. support XRD evidence of 1:1 clay species at upper depth. -

High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland
minerals Article High Temperature Sulfate Minerals Forming on the Burning Coal Dumps from Upper Silesia, Poland Jan Parafiniuk * and Rafał Siuda Faculty of Geology, University of Warsaw, Zwirki˙ i Wigury 93, 02-089 Warszawa, Poland; [email protected] * Correspondence: j.parafi[email protected] Abstract: The subject of this work is the assemblage of anhydrous sulfate minerals formed on burning coal-heaps. Three burning heaps located in the Upper Silesian coal basin in Czerwionka-Leszczyny, Radlin and Rydułtowy near Rybnik were selected for the research. The occurrence of godovikovite, millosevichite, steklite and an unnamed MgSO4, sometimes accompanied by subordinate admixtures of mikasaite, sabieite, efremovite, langbeinite and aphthitalite has been recorded from these locations. Occasionally they form monomineral aggregates, but usually occur as mixtures practically impossible to separate. The minerals form microcrystalline masses with a characteristic vesicular structure resembling a solidified foam or pumice. The sulfates crystallize from hot fire gases, similar to high temperature volcanic exhalations. The gases transport volatile components from the center of the fire but their chemical compositions are not yet known. Their cooling in the near-surface part of the heap results in condensation from the vapors as viscous liquid mass, from which the investigated minerals then crystallize. Their crystallization temperatures can be estimated from direct measurements of the temperatures of sulfate accumulation in the burning dumps and studies of their thermal ◦ decomposition. Millosevichite and steklite crystallize in the temperature range of 510–650 C, MgSO4 Citation: Parafiniuk, J.; Siuda, R. forms at 510–600 ◦C and godovikovite in the slightly lower range of 280–450 (546) ◦C. -

20210311 IAEG AD-DSL V5.0 for Pdf.Xlsx
IAEGTM AD-DSL Release Version 4.1 12-30-2020 Authority: IAEG Identity: AD-DSL Version number: 4.1 Issue Date: 2020-12-30 Key Yellow shading indicates AD-DSL family group entries, which can be expanded to display a non-exhaustive list of secondary CAS numbers belonging to the family group Substance Identification Change Log IAEG Regulatory Date First Parent Group IAEG ID CAS EC Name Synonyms Revision Date ECHA ID Entry Type Criteria Added IAEG ID IAEG000001 1327-53-3 215-481-4 Diarsenic trioxide Arsenic trioxide R1;R2;D1 2015-03-17 2015-03-17 100.014.075 Substance Direct Entry IAEG000002 1303-28-2 215-116-9 Diarsenic pentaoxide Arsenic pentoxide; Arsenic oxide R1;R2;D1 2015-03-17 2015-03-17 100.013.743 Substance Direct Entry IAEG000003 15606-95-8 427-700-2 Triethyl arsenate R1;R2;D1 2015-03-17 2017-08-14 100.102.611 Substance Direct Entry IAEG000004 7778-39-4 231-901-9 Arsenic acid R1;R2;D1 2015-03-17 2015-03-17 100.029.001 Substance Direct Entry IAEG000005 3687-31-8 222-979-5 Trilead diarsenate R1;R2;D1 2015-03-17 2017-08-14 100.020.890 Substance Direct Entry IAEG000006 7778-44-1 231-904-5 Calcium arsenate R1;R2;D1 2015-03-17 2017-08-14 100.029.003 Substance Direct Entry IAEG000009 12006-15-4 234-484-1 Cadmium arsenide Tricadmium diarsenide R1;R2;D1 2017-08-14 2017-08-14 Substance Direct Entry IAEG000021 7440-41-7 231-150-7 Beryllium (Be) R2 2015-03-17 2019-01-24 Substance Direct Entry IAEG000022 1306-19-0 215-146-2 Cadmium oxide R1;R2;D1 2015-03-17 2017-08-14 100.013.770 Substance Direct Entry IAEG000023 10108-64-2 233-296-7 Cadmium -

Carbonate, Sulfate and Phosphate Minerals Groups
University of Anbar Collage of Science Department of Geology Minerals / 1st stage. Carbonate, Sulfate and Phosphate Minerals Groups Assistant lecturer Nazar Zaidan Khalaf Carbonate, Sulfate and phosphate minerals groups Lecture seven Carbonate mineral group 2- • The carbonate minerals contain the anionic complex CO3 , ,which is triangular in its coordination—i.e., with a carbon atom at the center and an oxygen atom at each of the corners of an equilateral triangle. These anionic groups are strongly bonded individual units and do not share oxygen atoms with one another. The triangular carbonate groups are the basic building units of all carbonate minerals and are largely responsible for the properties particular to the class. • The common anhydrous (water-free) carbonates are divided into three groups that differ in structure type: calcite, aragonite, and dolomite. • The copper carbonates azurite and malachite are the only notable hydrous varieties. • This anion group usually occurs in combination with calcium, sodium, uranium, iron, aluminum, manganese, barium, zinc, copper, lead, or the rare-earth elements. The carbonates tend to be soft, soluble in hydrochloric acid, and have a marked anisotropy in many physical properties (e.g., high birefringence) as a result of the planar structure of the carbonate ion. • There are approximately 80 known carbonate minerals, but most of them are rare. The commonest varieties, calcite, dolomite, and aragonite, are prominent constituents of certain rocks: calcite is the principal mineral of limestone's and marbles; dolomite occurs as a replacement for calcite in limestone's, and when this is extensive the rock is termed dolomite; and aragonite occurs in some recent sediments and in the shells of organisms that have calcareous skeletons. -

CM300-011 Barium Chromate
Version: 1.0 Revision date: Safety Data Sheet 09/28/2015 Supersedes: 03/01/2013 Barium Chromate 1. PRODUCT AND COMPANY IDENTIFICATION 1.1. Product Identifiers Product Form: crystalline Substance Name: Barium Chromate CAS No.: 10294-40-3 Product Code: UIC, Inc. Catalog Number CM300-011 1.2. Intended Use of the Product Use of the substance/mixture: Laboratory chemicals, Manufacture of substances, Combustion tubes Name, Address, and Telephone of the Responsible Party UIC Inc 1225 Channahon Rd Joliet, IL 60436 Phone: (815) 744-4477 Fax: (815) 744-1561 Emergency Telephone Number For Chemical Emergency, Spill, Leak, Fire, Exposure, or Accident, call emergency number: 1-815-474-8753 2. Hazards Identification of the product 2.1. Classification of the substance or mixture Oxidizing solids (Category 2), H272 Acute toxicity, Oral (Category 4), H302 Acute toxicity, Inhalation (Category 4), H332 Carcinogenicity (Category 1A), H350 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2. GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H272 May intensify fire; oxidiser. H302 + H332 Harmful if swallowed or if inhaled H350 May cause cancer. Precautionary statement(s) P201 Obtain special instructions before use. P202 Do not handle until all safety precautions have been read and understood. P210 Keep away from heat. P220 Keep/Store away from clothing/ combustible materials. P221 Take any precaution to avoid mixing with combustibles. P261 Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray. P264 Wash skin thoroughly after handling. P270 Do not eat, drink or smoke when using this product.