Sparkling Feather Reflections of a Bird-Of-Paradise Explained by Finite-Difference Time-Domain Modeling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ORNITHOLOGIST VOLUME 44 - PARTS 1&2 - November - 2019
SOUTH AUSTRALIAN ORNITHOLOGIST VOLUME 44 - PARTS 1&2 - November - 2019 Journal of The South Australian Ornithological Association Inc. In this issue: Variation in songs of the White-eared Honeyeater Phenotypic diversity in the Copperback Quailthrush and a third subspecies Neonicotinoid insecticides Bird Report, 2011-2015: Part 1, Non-passerines President: John Gitsham The South Australian Vice-Presidents: Ornithological John Hatch, Jeff Groves Association Inc. Secretary: Kate Buckley (Birds SA) Treasurer: John Spiers FOUNDED 1899 Journal Editor: Merilyn Browne Birds SA is the trading name of The South Australian Ornithological Association Inc. Editorial Board: Merilyn Browne, Graham Carpenter, John Hatch The principal aims of the Association are to promote the study and conservation of Australian birds, to disseminate the results Manuscripts to: of research into all aspects of bird life, and [email protected] to encourage bird watching as a leisure activity. SAOA subscriptions (e-publications only): Single member $45 The South Australian Ornithologist is supplied to Family $55 all members and subscribers, and is published Student member twice a year. In addition, a quarterly Newsletter (full time Student) $10 reports on the activities of the Association, Add $20 to each subscription for printed announces its programs and includes items of copies of the Journal and The Birder (Birds SA general interest. newsletter) Journal only: Meetings are held at 7.45 pm on the last Australia $35 Friday of each month (except December when Overseas AU$35 there is no meeting) in the Charles Hawker Conference Centre, Waite Road, Urrbrae (near SAOA Memberships: the Hartley Road roundabout). Meetings SAOA c/o South Australian Museum, feature presentations on topics of ornithological North Terrace, Adelaide interest. -

Evolution of Correlated Complexity in the Radically Different Courtship Signals of Birds-Of-Paradise
bioRxiv preprint doi: https://doi.org/10.1101/351437; this version posted June 20, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Evolution of correlated complexity in the radically different courtship signals of birds-of-paradise 5 Russell A. Ligon1,2*, Christopher D. Diaz1, Janelle L. Morano1, Jolyon Troscianko3, Martin Stevens3, Annalyse Moskeland1†, Timothy G. Laman4, Edwin Scholes III1 1- Cornell Lab of Ornithology, 159 Sapsucker Woods Rd, Ithaca, NY, USA. 10 2- Department of Neurobiology and Behavior, Cornell University, Ithaca, NY 14853, USA. 3- Centre for Ecology and Conservation, College of Life and Environmental Science, University of Exeter, Penryn, Cornwall TR10 9FE, UK 4- Museum of Comparative Zoology, Harvard University, 26 Oxford St., Cambridge, MA 02138, USA 15 † Current address: Department of Zoology, University of Oxford, UK *Author for correspondence: [email protected] ORCID: Russell Ligon https://orcid.org/0000-0002-0195-8275 20 Janelle Morano https://orcid.org/0000-0001-5950-3313 Edwin Scholes https://orcid.org/0000-0001-7724-3201 [email protected] [email protected] 25 [email protected] [email protected] [email protected] [email protected] [email protected] 30 keywords: ornament, complexity, behavioral analyses, sensory ecology, phenotypic radiation 35 1 bioRxiv preprint doi: https://doi.org/10.1101/351437; this version posted June 20, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Management and Breeding of Birds of Paradise (Family Paradisaeidae) at the Al Wabra Wildlife Preservation
Management and breeding of Birds of Paradise (family Paradisaeidae) at the Al Wabra Wildlife Preservation. By Richard Switzer Bird Curator, Al Wabra Wildlife Preservation. Presentation for Aviary Congress Singapore, November 2008 Introduction to Birds of Paradise in the Wild Taxonomy The family Paradisaeidae is in the order Passeriformes. In the past decade since the publication of Frith and Beehler (1998), the taxonomy of the family Paradisaeidae has been re-evaluated considerably. Frith and Beehler (1998) listed 42 species in 17 genera. However, the monotypic genus Macgregoria (MacGregor’s Bird of Paradise) has been re-classified in the family Meliphagidae (Honeyeaters). Similarly, 3 species in 2 genera (Cnemophilus and Loboparadisea) – formerly described as the “Wide-gaped Birds of Paradise” – have been re-classified as members of the family Melanocharitidae (Berrypeckers and Longbills) (Cracraft and Feinstein 2000). Additionally the two genera of Sicklebills (Epimachus and Drepanornis) are now considered to be combined as the one genus Epimachus. These changes reduce the total number of genera in the family Paradisaeidae to 13. However, despite the elimination of the 4 species mentioned above, 3 species have been newly described – Berlepsch's Parotia (P. berlepschi), Eastern or Helen’s Parotia (P. helenae) and the Eastern or Growling Riflebird (P. intercedens). The Berlepsch’s Parotia was once considered to be a subspecies of the Carola's Parotia. It was previously known only from four female specimens, discovered in 1985. It was rediscovered during a Conservation International expedition in 2005 and was photographed for the first time. The Eastern Parotia, also known as Helena's Parotia, is sometimes considered to be a subspecies of Lawes's Parotia, but differs in the male’s frontal crest and the female's dorsal plumage colours. -

The Effect of Intense Light on Bird Behavior and Physiology
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@University of Nebraska University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Wildlife Damage Management, Internet Center Bird Control Seminars Proceedings for October 1973 THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY Sheldon Lustick Ohio State University Follow this and additional works at: https://digitalcommons.unl.edu/icwdmbirdcontrol Part of the Environmental Sciences Commons Lustick, Sheldon, "THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY" (1973). Bird Control Seminars Proceedings. 119. https://digitalcommons.unl.edu/icwdmbirdcontrol/119 This Article is brought to you for free and open access by the Wildlife Damage Management, Internet Center for at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Bird Control Seminars Proceedings by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. 171 THE EFFECT OF INTENSE LIGHT ON BIRD BEHAVIOR AND PHYSIOLOGY Sheldon Lustick Zoology Department Ohio State University , Columbus , Ohio 43210 It has been known for centuries that light (photoperiod ) is possibly the major environmental stimuli affecting bird behavior and physiology. The length of the light period stimulates the breeding cycle , migration , fat de - position , and molt in most species of birds. Therefore , it is only natural that one would think of using light as a means of bird control. In fa ct , light has already been used as a bird control; flood -light traps have been used to trap blackbirds (Meanley 1971 ); Meanley states that 2000 -W search lights have been used to alleviate depredation by ducks in rice fields. -

Birds and Language
BIRDS AND LANGUAGE Conference Thursday 19 and Friday 20 August 2021 9:00am – 5:00 pm Room 203 RD Watt Building Room, and Zoom The University of Sydney Pop-up event: Sydney College of the Arts, Building A22, Old Teacher’s College Birds and Language is supported by the Sydney College of the Arts, School of Literature, Art and Media, and the Sydney Environment Institute at the University of Sydney Image: Eugene Carchesio The Ventriloquist Series 1 (detail) 2017 Watercolour on paper © the artist and Milani Gallery, Brisbane. Photography by Madeleine Kelly. BIRDS AND LANGUAGE The sounds birds maKe form structured series, comprised of complex syntaxes, nuanced in tone, precise, sometimes excessive, often regarded as being of compelling aesthetic value. We do not hesitate to refer to many of these sounds as songs, or, more prosaically, calls. We move, easily, too, towards thinKing about these sounds as a species of language. More, we readily speaK of the visual rhetorics of birds: ideas of performativity, display, mimesis and deception. We sometimes dare to thinK of birds as artists—not only singers, but bricoleurs, assembling extravagant, colour-coded nests, as in the case of the bowerbird. More, recently, we have become more comfortable with thinKing of some birds as capable of higher-order reason, as experiments with crows demonstrate capacities to thinK through and to solve complex physical problems. This conference poses a simple question: What is it to talK of birds and language? How might such a question provide the impetus and grounds for an interdisciplinary encounter between the natural sciences, the humanities, and the creative arts? PROGRAMME Thursday 19 August 9:00 – 9:30 Madeleine Kelly Welcome and context Papers on birds, sky and materiality 9:30 – 10:00 David BrooKs Bird Song (extracts) Birdsong. -

Magnificent Magpie Colours by Feathers with Layers of Hollow Melanosomes Doekele G
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb174656. doi:10.1242/jeb.174656 RESEARCH ARTICLE Magnificent magpie colours by feathers with layers of hollow melanosomes Doekele G. Stavenga1,*, Hein L. Leertouwer1 and Bodo D. Wilts2 ABSTRACT absorption coefficient throughout the visible wavelength range, The blue secondary and purple-to-green tail feathers of magpies are resulting in a higher refractive index (RI) than that of the structurally coloured owing to stacks of hollow, air-containing surrounding keratin. By arranging melanosomes in the feather melanosomes embedded in the keratin matrix of the barbules. barbules in more or less regular patterns with nanosized dimensions, We investigated the spectral and spatial reflection characteristics of vivid iridescent colours are created due to constructive interference the feathers by applying (micro)spectrophotometry and imaging in a restricted wavelength range (Durrer, 1977; Prum, 2006). scatterometry. To interpret the spectral data, we performed optical The melanosomes come in many different shapes and forms, and modelling, applying the finite-difference time domain (FDTD) method their spatial arrangement is similarly diverse (Prum, 2006). This has as well as an effective media approach, treating the melanosome been shown in impressive detail by Durrer (1977), who performed stacks as multi-layers with effective refractive indices dependent on extensive transmission electron microscopy of the feather barbules the component media. The differently coloured magpie feathers are of numerous bird species. He interpreted the observed structural realised by adjusting the melanosome size, with the diameter of the colours to be created by regularly ordered melanosome stacks acting melanosomes as well as their hollowness being the most sensitive as optical multi-layers. -

The Molecular Evolution of Feathers with Direct Evidence from Fossils
The molecular evolution of feathers with direct evidence from fossils Yanhong Pana,1, Wenxia Zhengb, Roger H. Sawyerc, Michael W. Penningtond, Xiaoting Zhenge,f, Xiaoli Wange,f, Min Wangg,h, Liang Hua,i, Jingmai O’Connorg,h, Tao Zhaoa, Zhiheng Lig,h, Elena R. Schroeterb, Feixiang Wug,h, Xing Xug,h, Zhonghe Zhoug,h,i,1, and Mary H. Schweitzerb,j,1 aChinese Academy of Sciences Key Laboratory of Economic Stratigraphy and Palaeogeography, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China; bDepartment of Biological Sciences, North Carolina State University, Raleigh, NC 27695; cDepartment of Biological Sciences, University of South Carolina, Columbia, SC 29205; dAmbioPharm Incorporated, North Augusta, SC 29842; eInstitute of Geology and Paleontology, Lingyi University, Lingyi City, 27605 Shandong, China; fShandong Tianyu Museum of Nature, Pingyi, 273300 Shandong, China; gCAS Key Laboratory of Vertebrate Evolution and Human Origins of the Chinese Academy of Sciences, Institute of Vertebrate Paleontology and Paleoanthropology, Chinese Academy of Sciences, 100044 Beijing, China; hCenter for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, 100044 Beijing, China; iCollege of Earth and Planetary Sciences, University of Chinese Academy of Sciences, 100049 Beijing, China; and jNorth Carolina Museum of Natural Sciences, Raleigh, NC 27601 Contributed by Zhonghe Zhou, December 15, 2018 (sent for review September 12, 2018; reviewed by Dominique G. Homberger and Chenxi Jia) Dinosaur fossils possessing integumentary appendages of various feathers in Anchiornis, barbules that interlock to form feather morphologies, interpreted as feathers, have greatly enhanced our vanes critical for flight have not been identified yet (12). -
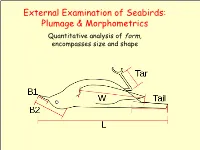
External Examination of Seabirds: Plumage & Morphometrics
External Examination of Seabirds: Plumage & Morphometrics Quantitative analysis of form, encompasses size and shape Seabird Topography ➢ Naming Conventions: • Parts of the body • Types of feathers (Harrison 1983) Types of Feathers Coverts: Rows bordering and overlaying the edges of the tail and wings on both the lower and upper sides of the body. Help streamline shape of the wings and tail and provide the bird with insulation. Feather Tracks ➢ Feathers are not attached randomly. • They occur in linear tracts called pterylae. • Spaces on bird's body without feather tracts are called apteria. • Densest area for feather tracks is head and neck. • Feathers arranged in distinct layers: contour feathers overlay down. Generic Pterylae Types of Feathers Contour feathers: outermost feathers. Define the color and shape of the bird. Contour feathers lie on top of each other, like shingles on a roof. Shed water, keeping body dry and insulated. Each contour feather controlled by specialized muscles which control their position, allowing the bird to keep the feathers in clean and neat condition. Specialized contour feathers used for flight: delineate outline of wings and tail. Types of Feathers Flight feathers – special contour feathers Define outline of wings and tail Long and stiff Asymmetrical those on wings are called remiges (singular remex) those on tail are called retrices (singular retrix) Types of Flight Feathers Remiges: Largest contour feathers (primaries / secondaries) Responsible for supporting bird during flight. Attached by ligaments or directly to the wing bone. Types of Flight Feathers Flight feathers – special contour feathers Rectrices: tail feathers provide flight stability and control. Connected to each other by ligaments, with only the inner- most feathers attached to bone. -

West Papua – Birds-Of-Paradise and Endemics of the Arfaks and Waigeo
INDONESIA: WEST PAPUA – BIRDS-OF-PARADISE AND ENDEMICS OF THE ARFAKS AND WAIGEO 03 – 14 AUGUST 2022 03 – 14 AUGUST 2023 Wilson’s Bird-of-paradise is often considered one of the best-looking birds in the world! www.birdingecotours.com [email protected] 2 | ITINERARY Indonesia: West Papua – Arfak and Waigeo New Guinea is a geographic rather than political term that refers to the main island in the region. The western half of the island of New Guinea comprises the Indonesian provinces of West Papua (Papua Barat) and Papua, collectively once called West Irian or Irian Jaya; the eastern half of the main island of New Guinea comprises the country of Papua New Guinea. We will be based in West Papua for this exhilarating, small-group birding adventure. Aside from the large landmass of New Guinea, the New Guinea region includes numerous small islands (some part of Indonesia and others part of Papua New Guinea), and we will visit one of these areas: Waigeo, part of the Raja Ampat Archipelago in West Papua (also known as the Northwestern Islands). Approximately 680 bird species have been recorded from West Papua, from slightly more than 700 for the whole New Guinea region. Some 550 species are considered breeding residents, with 279 New Guinea endemics (found in Indonesia and/or Papua New Guinea) and at least an additional 42 endemics found only in West Papua. There are also over 115 Palearctic and Australian migrant species and a range of seabirds which spend some of their time in West Papua. This tour will begin in the town of Manokwari, situated on the north-eastern tip of West Papua's Bird's Head (or Vogelkop) Peninsula where we could get our tour started with the gorgeous Lesser Bird-of-paradise, this area is usually great for Blyth’s Hornbill and numerous fruit doves. -

Short Communications Courtship Display and Mating of the Superb
Short Communications Courtship Display and Mating of the Superb Bird of Paradise Lophorina superba D.W. & C.B. FRITH "Prionodura':Paluma via Townsville, Queensland 4816 Emu 88, 183-188 Received 17 May 1987, accepted 9 October 1987 The Superb Bird of Paradise Lophorina superba inhabits December 1977 and November 1985 and we compared rainforest and forest edge between approximately 1000 these with those of the wild birds and descriptions by other and 2250 m above mean sea level (amsl) throughout authors. mainland New Guinea. The adult male is velvet black with metallic-like oil-green iridescent plumage on the crown, an Initial dkplay activily (IDA) erectile iridescent breast shield, and with a very long erectile and spreadable cape of modified velvet black nape This was observed seven times and consists of a sleeked feathers. Peculiar horn-like naral tufts of erectile rather stiff pose, held for about five seconds, before movement of black feathers adorn the base of the upper mandible, above cape, breast and naral tuft plumage. The male slightly the nostrils, as does a similar tuft of feathers beneath the crouches with breast shield sleeked tightly back against lower mandible. The female is cryptically coloured in himself, cape held back and down against the back, wings browns and greys with distinct blackish ventral bamng, and tail held normally, head and bill pointed upwards with like many other female paradisaeids (see Gilliard 1969; eyes fixed on the female, and naral tufts projecting conspic- Cooper & Forshaw 1977). First year male plumage is like uously forward and bifurcate (Fig. 1). This pose is followed that of the female, the black and iridescent plumage of by a repeated, sudden, upward and outward extension of adult males being acquired gradually over several years the breast shield, with head and bill still pointing at the (Gilliard 1969). -

2020 Sample (PDF)
® field guides BIRDING TOURS WORLDWIDE [email protected] • 800•728•4953 ITINERARY NEW GUINEA & AUSTRALIA October 10-28, 2020 One of the most amazing birds in New Guinea, a country full of amazing birds, is the Ribbon-tailed Astrapia. These birds- of-paradise are restricted to a small region in the central highlands. We should see them near Kumul Lodge, although we may not find one with a tail as extravagant as the one pictured here. Photograph by guide Doug Gochfeld. We include here information for those interested in the 2020 Field Guides New Guinea & Australia tour: ¾ a general introduction to the tour ¾ a description of the birding areas to be visited on the tour ¾ an abbreviated daily itinerary with some indication of the nature of each day’s birding outings These additional materials will be made available to those who register for the tour: ¾ an annotated list of the birds recorded on a previous year’s Field Guides trip to the area, with comments by guide(s) on notable species or sightings (may be downloaded from our web site) ¾ a detailed information bulletin with important logistical information and answers to questions regarding accommodations, air arrangements, clothing, currency, customs and immigration, documents, health precautions, and personal items ¾ a reference list ¾ a Field Guides checklist for preparing for and keeping track of the birds we see on the tour ¾ after the conclusion of the tour, a list of birds seen on the tour If you think you may have only one chance to visit Australasia, and you’re looking for a good sampling of the region’s unique wildlife, this tour is designed for you. -

Colour De Verre Molds: Feather
REUSABLE MOLDS FOR GLASS CASTING With either product, clean the See our website’s Learn section for mold with a stiff nylon brush and/ more instructions about priming or toothbrush to remove any old Colour de Verre molds with ZYP. kiln wash or boron nitride. (This step can be skipped if the mold is Filling the Feather brand new.) The suggested fill weight for the Feather is 330 to 340 grams. The If you are using Hotline Primo most simple way to fill the Feather Primer, mix the product according mold is to weigh out 330 of fine to directions. Apply the Primo frit and to evenly distribute the frit Primer™ with a soft artist’s brush in the mold. Fire the mold and frit (not a hake brush) and use a hair according the Casting Schedule Feather dryer to completely dry the coat. below. This design is also a perfect Create feathers that are as fanci- Give the mold four to five thin, candidate for our Wafer-Thin ful or realistic as you like with even coats drying each coat with a technique. One can read more Colour de Verre’s Feather de- hair dryer before applying the about this at www.colourdeverre.- sign. Once feathers are cast, next. Make sure to keep the Primo they can be slumped into amaz- com/go/wafer. ing decorative or functional well stirred as it settles quickly. pieces. The mold should be totally dry before filling. There is no reason to nnn pre-fire the mold. To use ZYP, hold the can 10 to 12 Feathers are a design element that inches from the mold.