April 5, 2021 – 2021.16
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
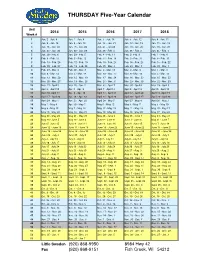
Thursday Calendar 20142018
THURSDAY Five-Year Calendar Unit 2014 20152016 2017 2018 Week # 1 Jan. 2 - Jan. 9 Jan. 1 - Jan. 8 Jan. 7 - Jan. 14 Jan. 5 - Jan. 12 Jan. 4 - Jan. 11 2 Jan. 9 - Jan. 16 Jan. 8 - Jan. 15 Jan. 14 - Jan. 21 Jan. 12 - Jan. 19 Jan. 11 - Jan. 18 3 Jan. 16 - Jan. 23 Jan. 15 - Jan. 22 Jan. 21 - Jan 28 Jan. 19 - Jan. 26 Jan. 18 - Jan. 25 4 Jan. 23 - Jan. 30 Jan. 22 - Jan. 29 Jan. 28 - Feb. 4 Jan. 26 - Feb. 2 Jan. 25 - Feb. 1 5 Jan. 30 - Feb. 6 Jan. 29 - Feb. 5 Feb. 4 - Feb. 11 Feb. 2- Feb. 9 Feb. 1 - Feb. 8 6 Feb. 6 - Feb. 13 Feb. 5 - Feb. 12 Feb. 11 - Feb. 18 Feb. 9 - Feb. 16 Feb. 8 - Feb. 15 7 Feb. 13 - Feb. 20 Feb. 12 - Feb. 19 Feb. 18 - Feb. 25 Feb. 16 - Feb. 23 Feb. 15 - Feb. 22 8 Feb. 20 - Feb. 27 Feb. 19 - Feb. 26 Feb. 25 - Mar. 3 Feb. 23 - Mar. 2 Feb. 22 - Mar. 1 9 Feb. 27 - Mar. 6 Feb. 26 - Mar. 5 Mar. 3 - Mar. 10 Mar. 2 - Mar. 9 Mar. 1 - Mar. 8 10 Mar. 6 - Mar. 13 Mar. 5 - Mar. 12 Mar. 10 - Mar. 17 Mar. 9 - Mar. 16 Mar. 5 - Mar. 15 11 Mar. 13 - Mar. 20 Mar. 12 - Mar. 19 Mar. 17 - Mar. 24 Mar. 16 - Mar. 23 Mar. 15 - Mar. 22 12 Mar. 20 - Mar. 27 Mar. 19 - Mar. 26 Mar. 24 - Mar. 31 Mar. 23 - Mar. 30 Mar. 22 - Mar. -

GOOGLE LLC V. ORACLE AMERICA, INC
(Slip Opinion) OCTOBER TERM, 2020 1 Syllabus NOTE: Where it is feasible, a syllabus (headnote) will be released, as is being done in connection with this case, at the time the opinion is issued. The syllabus constitutes no part of the opinion of the Court but has been prepared by the Reporter of Decisions for the convenience of the reader. See United States v. Detroit Timber & Lumber Co., 200 U. S. 321, 337. SUPREME COURT OF THE UNITED STATES Syllabus GOOGLE LLC v. ORACLE AMERICA, INC. CERTIORARI TO THE UNITED STATES COURT OF APPEALS FOR THE FEDERAL CIRCUIT No. 18–956. Argued October 7, 2020—Decided April 5, 2021 Oracle America, Inc., owns a copyright in Java SE, a computer platform that uses the popular Java computer programming language. In 2005, Google acquired Android and sought to build a new software platform for mobile devices. To allow the millions of programmers familiar with the Java programming language to work with its new Android plat- form, Google copied roughly 11,500 lines of code from the Java SE pro- gram. The copied lines are part of a tool called an Application Pro- gramming Interface (API). An API allows programmers to call upon prewritten computing tasks for use in their own programs. Over the course of protracted litigation, the lower courts have considered (1) whether Java SE’s owner could copyright the copied lines from the API, and (2) if so, whether Google’s copying constituted a permissible “fair use” of that material freeing Google from copyright liability. In the proceedings below, the Federal Circuit held that the copied lines are copyrightable. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

Monday, April 5, 2021
Monday, April 5, 2021 WHERE WE ARE TODAY 3 April 5, 2021 OUR PROGRESS ON VACCINATIONS 4 # DC residents partially or fully vaccinated January 1 5,846 February 1 40,839 March 1 74,811 April 1 162,669 (Apr 2) Data source: DC Health, using received data through 4/2/2021. Data subject to change. April 5, 2021 5 We have more work to do to get all residents vaccinated. Make sure you’re signed up to get your COVID-19 shot. Go to: Call: vaccinate.dc.gov 1-855-363-0333 April 5, 2021 PRE-REGISTRATION SNAPSHOT 6 Approximately 190,621 people have pre-registered for a vaccination appointment and are awaiting an appointment. April 5, 2021 7 If you pre-registered for a vaccination appointment but already received your vaccine elsewhere you can email [email protected] to remove yourself from the pre-registration list. April 5, 2021 NEW AND UPCOMING CLINICS 8 This week, the vaccine clinic at the Convention Center will expand through a partnership with Safeway. Appointment invitations will be sent on April 6. On Friday, April 9, a new high-capacity vaccination site will open at Arena Stage. The site will be operated in partnership with DC Health and MedStar Health. Appointments will be made through vaccinate.dc.gov and the District’s call center. April 5, 2021 THE PATH FORWARD FIVE FACTORS DETERMINING AN ACTIVITY’S RE-OPEN POTENTIAL 10 1. MASKS - Can and is the activity completed with or without wearing a mask? Highest Risk Unmasked, Indoors, Long Time, Very Dense 2. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -
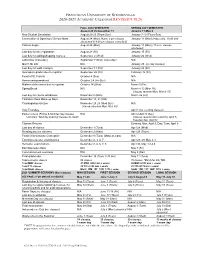
2020-2021 Academic Calendar Revised 9.18.20
FRANCISCAN UNIVERSITY OF STEUBENVILLE 2020-2021 ACADEMIC CALENDAR REVISED 9.18.20 FALL 2020 SEMESTER SPRING 2021 SEMESTER August 24 25-December 11 January 11-May 5 New Student Orientation August 20-23 (Thurs-Sun) January 7-10 (Thurs-Sun) Convocation & Opening of School Mass August 24 (Mon) (4 pm; 3 pm classes January 11 (Mon) (mass only, 10:30 am) shortened & 4:30 pm classes cancelled) Classes begin August 24 (Mon) January 11 (Mon) (10 a.m. classes shortened) Last day for late registration August 28 (Fri) January 15 (Fri) Last day for adding/dropping courses September 2 (Wed) January 20 (Wed) Labor Day (class day) September 7 (Mon) (class day) N/A March for Life N/A January 29 (no day classes) Last day for audit changes September 11 (Fri) January 22 (Fri) Incomplete grades due to registrar September 25 (Fri) February 12 (Fri) Feast of St. Francis October 4 (Sun) N/A Homecoming weekend October 2-4 (Fri-Sun) N/A Midterm deficiencies due to registrar October 14 (Wed) March 5 (Fri) Spring Break N/A March 8-12 (Mon-Fri) (classes resume Mon, March 15) Last day for course withdrawal November 2 (Mon) March 26 (Fri) Tentative Class Make-up Days November 14, 21 (Sat) Thanksgiving vacation November 25-29 (Wed-Sun) N/A (classes resume Mon, Nov 30) Holy Thursday April 1 (no evening classes) Easter recess (Friday & Monday day classes N/A April 2-April 5 (day) canceled; *Monday evening classes do meet) (classes resume Mon evening, April 5, Tuesday day, April 6) Classes Resume Evening: Mon, April 5; Day: Tues, April 6 Last day of classes December 1 (Tues) -

(Order List: 593 U.S.): Monday, April 5, 2021
(ORDER LIST: 593 U.S.) MONDAY, APRIL 5, 2021 ORDERS IN PENDING CASES 20M67 LYNN, MARY E. V. SAUL, COMM'R, SOCIAL SEC. The motion for leave to file a petition for a writ of certiorari under seal is denied. 19-251 ) AMERICANS FOR PROSPERITY V. RODRIQUEZ, ATT'Y GEN. OF CA ) 19-255 ) THOMAS MORE LAW CENTER V. RODRIQUEZ, ATT’Y GEN. OF CA The motion of petitioners for divided argument is denied. The motion of petitioners for enlargement of time for oral argument, and the motion of the Acting Solicitor General for leave to participate in oral argument as amicus curiae and for divided argument are granted, and the time is allotted as follows: 30 minutes for petitioners, 10 minutes for the Acting Solicitor General, and 30 minutes for respondent. 20-37 ) BECERRA, SEC. OF H&HS, ET AL. V. GRESHAM, CHARLES, ET AL. ) 20-38 ) ARKANSAS V. GRESHAM, CHARLES, ET AL. Upon consideration of the motion of petitioners to vacate the judgments of the court of appeals and remand, to remove the cases from the March 2021 argument calendar, and to hold further briefing in abeyance, these cases are held in abeyance pending further order of the Court. 20-255 MAHANOY AREA SCHOOL DISTRICT V. B. L. The motion of the Acting Solicitor General for leave to participate in oral argument as amicus curiae and for divided argument is granted. 1 20-334 SAN ANTONIO, TX V. HOTELS.COM, L.P., ET AL. The motion of the Acting Solicitor General for leave to participate in oral argument as amicus curiae and for divided argument is denied. -

Julian Date Cheat Sheet for Regular Years
Date Code Cheat Sheet For Regular Years Day of Year Calendar Date 1 January 1 2 January 2 3 January 3 4 January 4 5 January 5 6 January 6 7 January 7 8 January 8 9 January 9 10 January 10 11 January 11 12 January 12 13 January 13 14 January 14 15 January 15 16 January 16 17 January 17 18 January 18 19 January 19 20 January 20 21 January 21 22 January 22 23 January 23 24 January 24 25 January 25 26 January 26 27 January 27 28 January 28 29 January 29 30 January 30 31 January 31 32 February 1 33 February 2 34 February 3 35 February 4 36 February 5 37 February 6 38 February 7 39 February 8 40 February 9 41 February 10 42 February 11 43 February 12 44 February 13 45 February 14 46 February 15 47 February 16 48 February 17 49 February 18 50 February 19 51 February 20 52 February 21 53 February 22 54 February 23 55 February 24 56 February 25 57 February 26 58 February 27 59 February 28 60 March 1 61 March 2 62 March 3 63 March 4 64 March 5 65 March 6 66 March 7 67 March 8 68 March 9 69 March 10 70 March 11 71 March 12 72 March 13 73 March 14 74 March 15 75 March 16 76 March 17 77 March 18 78 March 19 79 March 20 80 March 21 81 March 22 82 March 23 83 March 24 84 March 25 85 March 26 86 March 27 87 March 28 88 March 29 89 March 30 90 March 31 91 April 1 92 April 2 93 April 3 94 April 4 95 April 5 96 April 6 97 April 7 98 April 8 99 April 9 100 April 10 101 April 11 102 April 12 103 April 13 104 April 14 105 April 15 106 April 16 107 April 17 108 April 18 109 April 19 110 April 20 111 April 21 112 April 22 113 April 23 114 April 24 115 April -
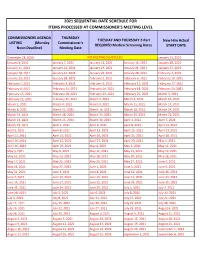
2021 Sequential Date List
2021 SEQUENTIAL DATE SCHEDULE FOR ITEMS PROCESSED AT COMMISSIONER'S MEETING LEVEL COMMISSIONERS AGENDA THURSDAY TUESDAY AND THURSDAY 2-Part New Hire Actual LISTING (Monday Commissioner's REQUIRED Medical Screening Dates START DATE Noon Deadline) Meeting Date December 28, 2020 NO MEETING SCHEDULED January 13, 2021 January 4, 2021 January 7, 2021 January 12, 2021 January 14, 2021 January 20, 2021 January 11, 2021 January 14, 2021 January 19, 2021 January 21, 2021 January 27, 2021 January 18, 2021 January 21, 2021 January 26, 2021 January 28, 2021 February 3, 2021 January 25, 2021 January 28, 2021 February 2, 2021 February 4, 2021 February 10, 2021 February 1, 2021 February 4, 2021 February 9, 2021 February 11, 2021 February 17, 2021 February 8, 2021 February 11, 2021 February 16, 2021 February 18, 2021 February 24, 2021 February 15, 2021 February 18, 2021 February 23, 2021 February 25, 2021 March 3, 2021 February 22, 2021 February 25, 2021 March 2, 2021 March 4, 2021 March 10, 2021 March 1, 2021 March 4, 2021 March 9, 2021 March 11, 2021 March 17, 2021 March 8, 2021 March 11, 2021 March 16, 2021 March 18, 2021 March 24, 2021 March 15, 2021 March 18, 2021 March 23, 2021 March 25, 2021 March 31, 2021 March 22, 2021 March 25, 2021 March 30, 2021 April 1, 2021 April 7, 2021 March 29, 2021 April 1, 2021 April 6, 2021 April 8, 2021 April 14, 2021 April 5, 2021 April 8, 2021 April 13, 2021 April 15, 2021 April 21, 2021 April 12, 2021 April 15, 2021 April 20, 2021 April 22, 2021 April 28, 2021 April 19, 2021 April 22, 2021 April 27, 2021 April -

Implementation Order
THE JUDICIAL COUNCIL OF THE STATE OF CALIFORNIA Because of the COVID-19 epidemic, leading to health and safety concerns resulting in the temporary cessation of jury services and substantial operational impediments, and the proclamation of a state of emergency by federal, state, and local officials, it has been determined that the conditions described in section 68115 of the Government Code are met with regard to the Superior Court of San Francisco County. Upon the request of Presiding Judge Garrett Wong, it is ordered that the Superior Court of San Francisco County is authorized to do the following: Extend by not more than 21 days the duration of any temporary restraining order that would otherwise expire from March 16, 2020 to April 15, 2020 inclusive, because the emergency condition described herein prevented the court from conducting proceedings to determine whether a permanent order should be entered (Gov. Code, § 68115(a)(7)); Extend the time period provided in section 825 of the Penal Code within which a defendant charged with a felony offense must be taken before a magistrate from 48 hours to not more than 7 days, applicable only to cases in which the statutory deadline otherwise would from March 16, 2020 to April 15, 2020 inclusive, (Gov. Code, § 68115(a)(8)); Extend the time period provided in section 859b of the Penal Code for the holding of a preliminary examination from 10 court days to not more than 15 court days, applicable only to cases in which the statutory deadline otherwise would expire from March 16, 2020 to April 15, 2020 inclusive, (Gov. -

Resolution No.: 2021-______
RESOLUTION NO.: 2021-______ WHEREAS, the Mayor and Council of the City of Salem met from time to time in Executive Session for purposes authorized by the Illinois Open Meetings Act; and WHEREAS, as required by the Act, the City Clerk has kept written minutes of all such executive sessions; and WHEREAS, pursuant to the requirements of Public Act 85-1355, the Mayor and Council have met in closed session to review all closed session minutes; and WHEREAS, they have determined that a need for confidentiality still exists as to the executive session minutes from the meetings set forth on Schedule "A" attached hereto; and WHEREAS, they have further determined that the minutes of the meetings listed on Schedule "B" attached hereto no longer require confidential treatment and should be made available for public inspection; NOW, THEREFORE, BE IT RESOLVED BY THE MAYOR AND COUNCIL OF THE CITY OF SALEM, MARION COUNTY, ILLINOIS, as follows: SECTION 1: The Executive Session Minutes from those meetings set forth on Schedule "B" attached hereto are hereby released. SECTION 2: The City Clerk is hereby authorized and directed to make said minutes available for inspection and copying in accordance with the standing procedures of the Clerk's Office. 1 SECTION 3: This Resolution shall be in full force and effect from and after its passage. IN WITNESS WHEREOF, I have hereunto set my hand and caused the seal of the City of Salem, Illinois, to be affixed this 6th day of July, 2021. CITY OF SALEM, ILLINOIS MAYOR ATTEST: CITY CLERK 2 SCHEDULE A EXECUTIVE SESSION