DWV Infection and Replication at the Early Stage in Vitro Using Honey Bee Pupal Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Niccolo Suzhou Opens 1 April 2021 As the Beacon of the Future in China's
For Immediate Release NICCOLO SUZHOU OPENS 1 APRIL 2021 AS THE BEACON OF THE FUTURE IN CHINA’S ‘VENICE OF THE EAST’ The Highly-Anticipated Niccolo Suzhou is the Fifth Iconic Landmark in China for Contemporary Chic Niccolo Hotels. 25 February 2021 (Suzhou, China) – Situated sky-high atop the magnificent new Suzhou International Finance Square (IFS) overlooking the city’s picturesque canals and Jinji lake, Niccolo Suzhou is set to become the most prestigious new luxury hotel address when it opens in April. The new Suzhou landmark towers majestically above the city - a shimmering glass tower shaped like a fish tail, which was designed as an auspicious tribute to the rivers and canals of the city known as “the Venice of the East”. It was designed by internationally-renowned architects Kohn Pederson Fox, whose other iconic global landmarks include Roppongi Hills in Tokyo, super towers Lotte World Tower in Seoul, Shanghai World Financial Centre and Hong Kong’s International Commerce Centre. Dubbed “The Beacon of the Future”, the new tower symbolises longevity and prosperity for Suzhou, now one of China’s top ten most affluent cities. Niccolo Suzhou, whose lobby soars 115 floors above the clouds, is its crowning glory, and the most hotly anticipated hotel to open in China this year. Taking inspiration from luxury fashion, Niccolo Hotels has been setting new benchmarks in contemporary chic lifestyles since the brand was formed in 2015. Niccolo Suzhou’s sister hotels in Changsha, Chengdu, Chongqing, and Hong Kong are recognised as epicentres of events and sophisticated occasions. Niccolo Suzhou Pre-Opening Office – Tower 1, Suzhou IFS, 409, Suzhou Avenue East, Suzhou Industrial Park, Suzhou, Jiangsu 215021, Chinaa niccolohotels.cn | niccolohotels.com 1 Each Niccolo hotel has quickly become the market leader in every city where it is located, transforming both the physical landscape and the cosmopolitan fabric of the city, and Niccolo Suzhou wlll be no exception. -
Annual Report
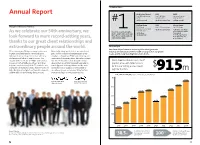
Top Ranking Report Annual Report Architectural Record ENR VMSD Top 300 Architecture Top 150 Global Top Retail Design Firms: Design Firms: Firms of 2014: # #1 Firm Overall #1 Architecture Firm #1 Firm Overall Building Design ENR Interior Design Message from the Board of Directors 2014 World Top 500 Design Firms: Top 100 Giants: Architecture 100 Most #1 Architecture Firm #1 Architecture Firm Admired Firms: Gensler is1 a leader among the #1 in Corporate Office As we celebrate our 50th anniversary, we world’s architecture and design #1 US Firm #1 in Retail #4 Global Firm #1 in Transportation firms. Here’s how we ranked in #1 in Government look forward to more record-setting years, our industry in 2014. #1 in Cultural thanks to our great client relationships and extraordinary people around the world. Financial Report Our financial performance and recognition throughout the We’re entering our 50th year stronger than ever. Financially strong and debt-free, we contributed industry are indications of the breadth of our practice, our global In 2014, our global growth continued apace $38.5 million in deferred compensation to our reach, and the long-standing trust of our clients. with our clients as they entrusted us with new employees through our ESOP, profit-sharing, and challenges and led us to new locations. Our international retirement plans. We made strategic expanded Gensler team of 4,700+ professionals investments in our research and professional We’ve broadened our services to 27 now work from 46 different offices. With their development programs, along with upgrades to practice areas, with total revenues help, we completed projects in 72 countries and our design-and-delivery platform and the tools for the year setting a new record $ increased our revenues to $915 million—a record and technology to support it. -

Suzhou Zhongnan Center: Rising Above Engineering Challenges 3
ctbuh.org/papers Title: Suzhou Zhongnan Center: Rising Above Engineering Challenges Author: Dong Shen, General Manager, Zhongnan Group Subjects: Architectural/Design Building Case Study Keywords: Geotechnical Engineering Megatall MEP Mixed-Use Performance Based Design Structural Engineering Vertical Transportation Publication Date: 2014 Original Publication: CTBUH 2014 Shanghai Conference Proceedings Paper Type: 1. Book chapter/Part chapter 2. Journal paper 3. Conference proceeding 4. Unpublished conference paper 5. Magazine article 6. Unpublished © Council on Tall Buildings and Urban Habitat / Dong Shen Suzhou Zhongnan Center: Rising Above Engineering Challenges 苏州中南中心大厦: 攻克工程难题 Abstract Suzhou is only 30 minutes by train from Shanghai, but has a rich history and identity all its own, soon to be augmented by the Zhongnan Tower, a 700-meter structure that could become the world’s tallest building outside the Middle East. The so-called “Venice of China” is moving into the 21st century, with the Zhongnan Tower anchoring a large urban development project, Suzhou Industrial Park. Dong Shen Keywords: Suzhou Zhongnan Tower, Suzhou Zhongnan Center, Megatall, 7-Star Hotel, Suzhou Industrial Park Dong Shen Zhongnan Group Suzhou Industrial Park, Su Ya Road, No. 308, Suzhou Letter Bldg. 11th Floor, Souzhou, China 215122 摘要 tel (电话): 86-13802885501 从苏州乘火车仅需30分钟便可抵达上海,但苏州这座城市拥有丰富的历史历史和独特的 fax (传真): 86-0755-82726306 email (电子邮箱): [email protected] 自我特征,而即将竣工的中南中心将进一步强化其特征,大楼具有700m高的结构,有 可能成为中东地区以外世界上最高的建筑。中南中心的建设奠定了一个大型城市开发项 Dong Shen received a bachelor’s degree from 目——苏州工业园区的发展,被称为“中国威尼斯”的苏州正在大步迈向二十一世纪。 Shanghai Jiaotong University, and then attended the Honolulu University, receiving a master’s degree in 关键词:苏州中南中心,苏州中南中心,巨型结构,七星级酒店,苏州工业园 business administration. He has many years’ experience of leadership in the real-estate industry. -

International Student Welcome Guide 2017-18 WELCOME to XJTLU 西浦欢迎你
XJTLU InternatIonal Student Welcome GuIde 2017-18 WELCOME TO XJTLU 西浦欢迎你 Thank you for choosing Xi’an Jiaotong - Liverpool University for a unique and rewarding learning experience. In order to make your transition to China as smooth as possible, we already started to prepare for your arrival. As part of our preparation, this booklet is specifically designed for you as a guide to ensure that everything goes to plan. Therefore, we strongly recommend you to spare enough time reading it carefully and act accordingly. Meanwhile, please feel free to contact us should you have any further enquiries. Many thanks to those who helped to review this guide. Every effort has been made to ensure the accuracy of the XJTLU International Student Welcome Guide 2017-18 Student Welcome International XJTLU information in this booklet, which is to be correct at the time of publication. XJTLU International Student Welcome Guide 2017-18 Student Welcome International XJTLU 2 3 CONTENTS KEY CONTACT INFORMATION 联系我们 6 METRO地铁 32 PRE-DEPARTURE TO DO LIST 行前待办 6 TAXIS的士 32 BEFORE YOU GO 行前准备 7 BICYCLES 自行车 34 ACCEPTING YOUR OFFER 录取通知书 7 E-BIKES 电动车 34 HOW TO PAY YOUR FEES 如何付学费 7 TRAVELLING IN CHINA 旅行 35 COST OF LIVING生活费 9 PLANE 飞机 35 VISAS 签证 11 TRAIN 火车 35 HEALTH 体检 13 COACHES大巴 36 WHAT TO PACK 行李 13 STUDENT WELLBEING 身心健康 37 XJTLU International Student Welcome Guide 2017-18 Student Welcome International XJTLU ELECTRONICS 电器 14 STUDENT COUNSELLING 心理咨询 37 WEATHER 天气 14 HOSPITALS 医院 37 VACCINATIONS 疫苗 14 RELIGION 宗教 38 MEDICATIONS 药品 15 SAFETY 安全第一 39 INSURANCE -

The Wharf (Holdings) Limited ANNUAL REPORT 2013
THE WHARF ( HOLDINGS ) LIMITED www.wharfholdings.com ANNUAL REPORT 2013 REPORT ANNUAL The Wharf (Holdings) Limited ANNUAL REPORT 2013 Stock Code: 4 With the opening of Chengdu IFS, Wharf has built another Harbour City in Chengdu. Strategically located at the intersection of three major commercial roads – Hongxing Road, Dacisi Road and Jiangnanguan Street, the 210,000-square-metre retail landmark also marked the maiden anchor of 100 world’s most coveted brands. Corporate Profile Backed by a long standing mission of ”Building for Tomorrow” and a distinguished track record, the Group has produced consistent and quality growth over the years. Wharf is among the top local blue chip stocks that are most actively traded, signalling high liquidity and attractiveness for investors. In addition, through years of value creation and new investment, the Group’s investment properties (“IP”) portfolio, with a book value of HK$261 billion as at the end of 2013, has grown to rank among the top five publicly-held portfolios in the world. It represented 70% of the Group’s total operating profit. With prime real estate as the Group’s primary strategic focus, site acquisition, financing, development planning, design, construction and marketing are its core competencies. Mall development and retail management remain its strategic differentiation. With its leadership in retail management, the Group continued to maintain its pole position in the Hong Kong retail market. Harbour City and Times Square, the Group’s landmark properties, had a combined value of HK$192 billion at the end of 2013, up from HK$177 billion in 2012, and represented 49% of the Group’s business assets. -

A JOURNEY at the BEACON of the FUTURE Niccolo Suzhou Grand Recruitment Offers Exciting Career Prospects
For Immediate Release A JOURNEY AT THE BEACON OF THE FUTURE Niccolo Suzhou Grand Recruitment Offers Exciting Career Prospects Crowning the top floors of Suzhou International Square (IFS), the city’s tallest skyscraper and Beacon of the Future. 12 December 2020 (Suzhou, China) – Suzhou, fondly known as China’s Venice of the East, will take centre stage as the city awaits the launch of a new iconic landmark, the Beacon of the Future and location of the contemporary chic hotel, Niccolo Suzhou, in 2021. The hotel recently held its eagerly-anticipated Grand Recruitment Event, becoming quickly the talk of the town and the epicentre of excitement in the industry. The Grand Recruitment Event was set against the Suzhou skyline in a fashionable and contemporary chic manner. Applicants from Suzhou and all over the country were eager to participate and experience the brand culture and talent cultivation of Niccolo Hotels. In order to provide a convenient and unforgettable experience of Niccolo Suzhou, the event included an online interview channel to allow for applications of candidates from outside of Suzhou. Niccolo Suzhou Pre-Opening Office – Tower 1, Suzhou IFS, 409, Suzhou Avenue East, Suzhou Industrial Park, Suzhou, Jiangsu 215021, China niccolohotels.cn | niccolohotels.com 1 Niccolo Suzhou Grand Recruitment Offers Exciting Career Prospects “At Niccolo Hotels, we stand for integrity, meticulousness, passion, respect, excellence, sincerity and sustainability. We look for talent not only capable to provide hospitality service, but reflecting the emotional intelligence to understand others. They have outgoing personalities and most importantly a high CQ [Cool Quotient] representing them as creative, dynamic, eloquent and forward thinking," said Mr. -

Capitamalls Asia and SIPJUD Sign Agreement to Develop Largest Shopping Mall in Suzhou Renowned Architectural Firm Benoy Appointed to Design the Development
The Singapore Exchange Securities Trading Limited, Hong Kong Exchanges and Clearing Limited and The Stock Exchange of Hong Kong Limited take no responsibility for the contents of this news release, make no representation as to its accuracy or completeness and expressly disclaim any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the contents of this news release. For immediate release 25 October 2011 NEWS RELEASE CapitaMalls Asia and SIPJUD sign agreement to develop largest shopping mall in Suzhou Renowned architectural firm Benoy appointed to design the development Singapore and Hong Kong, 25 October 2011 – CapitaMalls Asia Limited (SGX: JS8 and HKEx: 6813) signed a co-operation agreement with Suzhou Industrial Park Jinji Lake Urban Development Co., Ltd (“SIPJUD”) yesterday to develop the largest shopping mall in Suzhou. Owned by the Suzhou Industrial Park (“SIP”) government, SIPJUD is the master developer of the SIP Central Business District (“CBD”), known as the Suzhou Centre. The agreement was signed by Mr Lim Beng Chee, CEO of CapitaMalls Asia; and Mr Xu Guoping, President of SIPJUD during the 5 th Singapore-Jiangsu Co-operation Council (“SJCC”) conference in Singapore. The signing was witnessed by SJCC co-chairmen Mr Heng Swee Keat, Singapore’s Minister for Education; and Mr Li Xueyong, Governor of Jiangsu Province in China. Under the agreement, CapitaMalls Asia and SIPJUD will jointly develop and own a shopping mall and two office towers on the prime site in Suzhou Centre. The development, with a total gross floor area (“GFA”) of about 460,000 square metres (“sq m”), is in the heart of the western CBD of SIP, next to the renowned Jinji Lake and near the traditional city centre in Suzhou. -

China Propertiesproperties
ChinaChina PropertiesProperties NovemberNovember 28,28, 20072007 Disclaimer Wheelock (Stock code: 20) & Wharf (Stock code: 4) Group Presentation Disclaimer All information and data are provided for information purposes only. All opinions included herein constitute Wheelock and Wharf’s judgment as of the date hereof and are subject to change without notice. The Group, its subsidiaries and affiliates hereby disclaim (i) all express, implied, and statutory warranties of any kind to user and/or any third party including warranties as to accuracy, timeliness, completeness, or fitness for any particular purpose; and (ii) any liability whatsoever for any loss howsoever arising from or in reliance upon the whole or any part of the information and data contained herein. China Properties 2 Presentation Outline Introduction Flagship Projects - Shanghai Wheelock Square, Shanghai - Wuxi Super Tower, Wuxi - Suzhou Super Tower, Suzhou - Hongxing Lu, Chengdu 。IFC 。IFC Tiandi Residential Projects China Properties - Wholly-owned -J/V Outlook 3 China Macro Economic Environment Government revenue rose by 30% Vs last year Foreign exchange reserves ~ US$1,400B at Q3 2007 Low foreign debts ~ about 12% of GDP as at end of 2006 Inflation rate ~ 4.4% (Jan – Oct 2007) China Properties 4 Market Outlook China Property Market Robust economic growth and steady wealth creation process with increasing domestic demand in housing in China → continue to benefit its real estate developments Robust economic growth – 8-9% GDP growth p.a. and even higher in certain cities (11% in 2007) Growth in private enterprise formation ~ over 16% for 2006, ie. about 5 million private enterprises in mainland now (for 10-year up to 2005, the annual growth rate was about 30%!) Growth in automobile sales ~ over 20% p.a. -

The Building of a Chinese Model New Town: Case Study of the Suzhou Industrial Park
The Building of a Chinese Model New Town: Case Study of the Suzhou Industrial Park China has been undergoing rapid urbanization in the last three decades, with the percentage of urban population surging from 20.4% in 1982 to 52.6% in 2013. The trend continues with more than sixteen million rural residents move to urban area each year in what geographer David Harvey regards as ‘the largest mass migration the world has ever seen.’1 ZHONGJIE LIN The massive urbanization has resulted in unprecedented construction boom University of North Carolina and generated numerous new towns across the country. At the beginning of the at Charlotte twenty-first century, Chinese government announced that they would build 20 new cities each year in the next 20 years; therefore approximately 400 new cit- ies would emerge by 2020.2 These ambitious new town projects were not only created to house the swelling population, but also to sustain economic growth in the major cities they serve. Recognizing its enormous impact on Chinese soci- ety both in terms of challenge and opportunity, Premier Keqiang Li highlighted ‘urbanization’ as the keyword of economic restructuring in his political agenda after he took the post in 2013, calling for a more sustainable approach to the country’s mass urbanization to create new venues for jobs, consumptions, and investments, to balance mega-cities with small towns, and to correct economic disequilibrium between coastal and inland regions. The pursuit of a sustainable path of urbanization in China has actually accompa- nied the growth of urban population in the last two decades. -

NONEAD Shelf Carrier System CB4 Brochure
NONEAD Shelf Carrier System CB4 Brochure Document Version: 1.0.06171 Updated Date: 2020/6/17 Software Version 4.1.0 Suzhou Nonead Robot S&T Co., LTD. Address: Room A1101, International Science Park Phase Four, No.1355 Jinji Lake Avenue, Suzhou Industrial Park, Jiangsu Province, China. Http://www.nonead.com Http://www.cobotchina.com Email:[email protected] Tel :+86 18015500178 +86: 512 68630178 68630188 Fax: +86 512 68630198 Ext. 8008 Technical parameters are subject to change without prior notice New version download:https://www.nonead.com/en/hardware_content/20706.html ICS CODE:NH0145 NO. DOC1911010000671-3 n NONEAD Shelf Carrier System CB4 MiR-robots is a kind of indoor industrial cooperative autonomous mobile robot, which can realize the automation of material transportation in the factory, improve the speed of internal logistics, and liberate the employees from the repetitive and dangerous transportation work, so as to ensure the safety and efficiency of employees and robots, and improve productivity. MiR-robots has the characteristics of rapid arrangement, simple programming, safety and reliability, and does not need to change the working environment layout. MiR-robots enables user to adapt the changing market demands, new products and new production processes. Users can easily replace top modules, modify tasks, and add new features without external integration services. The fourth generation of NONEAD Shelf Carrier System is a multi-functional bottom traction system customized by NONEAD technology for MiR-robots. As the top standard module of MiR-robots, this system has the characteristics of fast installation, no need for additional programming, safety and reliability. -

Analysis and Enlightenment of Sports Competitions in Suzhou
Journal of Frontiers in Sport Research DOI: 10.23977/jfsr.2021.010110 Clausius Scientific Press, Canada Volume 1, Number 1, 2021 Analysis and Enlightenment of Sports Competitions in Suzhou Xiangbo Meng Suzhou Vocational University, Suzhou 215104, China Email: [email protected] Keywords: Suzhou, Folk sports events, Case study, Enlightenment Abstract: This article uses case analysis to explain the development process and development status of the typical cases of sports events among citizens in Suzhou-Suzhou Jinji Lake Dragon Boat Race and Suzhou Folk Basketball League, and it also analyzes and studies the enlightenment brought by the successful development of these two typical events, in order to provide references for the development of folk sports events in other regions in China. 1. Introduction Folk sports events refer to folk sports competitions held among certain people. It not only refers to some traditional folk sports events, but also refers to various mass modern sports events spontaneously developed by the people (Wang, Wu& Liu, 2016). In recent years, the number of sports events held among the citizens of Suzhou has shown a blowout trend. However, in the course of its gradual development, some new problems have also emerged, such as lack of brand events and insufficient media promotion, these become a factor hindering the development of sports events among the citizens of Suzhou. The Suzhou Jinji Lake Dragon Boat Race and the Suzhou Folk Basketball League are typical cases of the successful development of folk sports events, and their successful development experience has important guiding value for the development of other folk sports events in Suzhou. -

Suzhou, China: Jinji Lake CBD Hotel Market
Singapore: Hotel Market Market Report - March 2019 MARKET REPORT Suzhou, China: Jinji Lake CBD Hotel Market NOVEMBER 2019 Suzho, China: Jinji Lake CBD Hotel Market Market Report - November 2019 Introduction After the successful development over the past 20 years, As a historical tourism city located in the central area of the it has become a major contributor of the city’s GDP and Yangtze River Delta in Southern Jiangsu Province, Suzhou continues to progress and immerse into a core CBD area by is renowned for its classical Chinese gardens and canals, integrating a comprehensive array of modern city functions recognized as the ‘Venice of the East’. Over the last few such as grade-A offices, mix-use complexes, mega shopping decades, Suzhou has been able to attract a solid foundation malls as well as state-of-the-art research and development of foreign investments with its mature manufacturing centres. industry. The industry structural optimization and upgrade which happened over the last five years is considered a The hotel market we are reviewing in this report is factor that continues to drive the city’s economic growth. represented by major upscale and upper-upscale hotels found within the Jinji Lake CBD area. The hotel Specifically, advanced manufacturing such as: performance of this market is indicative of the overall semiconductor manufacturing, artificial intelligence, upscale and upper-upscale hotel market performance for new information, nano technology, biopharmaceutical Suzhou City as a whole. engineering and new materials are some key areas that contribute to the increased industrial outputs in the city Opening Year/ Room Hotel and gradually replaced many traditional heavily-polluted Renovation Count manufacturing factories.