A1132-C451-003-Jpeg.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Database of Mineral Resources of Zambia
International Workshop on UNFC-2009 DATABASEDATABASE OFOF MINERALMINERAL – Theory and Practice RESOURCESRESOURCES OFOF ZAMBIAZAMBIA Warsaw, 21-22 June 2010 By Prof. Imasiku Nyambe & Cryton Phiri University of Zambia, Geology Department, Lusaka-Zambia PRESENTATIONPRESENTATION LAYOUTLAYOUT y Introduction y Regional Geological Setting y Stratigraphy y Geology and Mineral Resources y Mining Administration Of Mineral Resource y Regional Mapping y Geochemical Survey y Mineral Resource Exploration y Mineral Resource Evaluation y Mineral Potential y Conclusion y References IntroductionIntroduction y Zambia is a landlocked country in Southern Africa with a total area of 752,614km² and with a population of 12 million people. Located well in the tropics and enjoys a sunny climate with three distinct seasons. The country is endowed with mineral resources and since 1930s the mining industry has been the economic backbone of Zambia. RegionalRegional GeologicalGeological SettingSetting y Zambia is a vivacious country forming a natural hub for the regions diverse activities. Its diverse mineral endowment is entirely a function of the variety of geological terrains and the multiplicity of thermal tectonic events that have overprinted and shaped these terrains. Position of Zambia RegionalRegional GeologicalGeological SettingSetting Zambia’s geological terrains y The multiplicity of tectono‐thermal events reflect somewhat a complex geology. These differential movements have played an important role in the geological evolution and the genesis of the country’s -

Status, Priorities and Needs for T I Bl Il T I Sustainable Soil Management In
Status, priorities and needs for sustitaina ble so il managemen tit in Zambia SSStalin Sichinga Zamb ia Ag ricu ltu re Resea r ch Institute Introduction Zambia has an area of 750,000 km2 with about 13.9 million people and ample land resources 0ut of 9 million ha cultivable land, only 14% is cropped in any year About 55 - 60% of the land area is covered by natural forest and 6% of Zambia‘s land surface is covered by water. Agro-ecological regions and soil distribution The country is classified into three agro-ecological regions based on soil types, rainfall, and other climatic conditions Agro-Ecological Regions N Chiengi Kaputa Mpulungu W E Nchelenge Mbala Nakonde Mporokoso S Kawambwa Mungwi Isoka Scale 1: 2,500,000 Mwense Luwingu Kasama Chinsali Chilubi Mansa Chama LEGEND Samfya Milenge Mpika Regions Mwinilunga Chililabombwe Solwezi Agro-ecological Region I Chingola Mufulira Lundazi I Ka lul u shi Kitwe Ndola IIa Lufwanyama Luans hya Chavuma Serenje Mambwe Kabompo Masaiti IIb Mpongwe Zambezi Mufumbwe Chipata Kasempa Petauke Katete Chadiza III Annual rainfall is <750mm Kapiri Mposhi Mkushi Nyimba Kabwe Lukulu Kaoma Mumbwa Chibombo Kalabo Mongu Chongwe Lusaka Urban Luangwa Itezhi-Tezhi Kafue Namwala Mazabuka Senanga Monze KEY Siavonga Sesheke Gwembe Shangombo Choma District boundary e Kazungula Kalomo w g n o z a in Livingstone S 200 0 200 400 Kilometers December 2002 The region contains a diversity of soil types ranging from slightly acidic Nitosols to alkaline Luvisols with pockets of Vertisols, Arenosols, Leptosols and, Solonetz. The physical limitations of region I soils Hazards to erosion, lim ite d so il dept h in t he hills an d escarpment zones, presence of hardpans in the pan dambo areas, ppyoor workability in the cracking gy, clay soils, problems of crusting in most parts of the Southern province, low water-holding capacities and the problem of wetness in the valley dambos, plains and swamps. -

US Embassy Lusaka American Citizen Services
U.S. Embassy Lusaka American Citizen Services Funeral and Repatriation Options In Zambia (updated Feb. 28, 2020) The death of an American citizen abroad can be a difficult and traumatic event. This brochure provides an insight to the funeral and repatriation services available in Zambia. Zambia is a Christian nation that is tolerant of all other religions. Most morgues follow Christian rites and practices with their burial preparations. In situations where one’s religion is not established at the time of death, Christian rituals would be performed for the deceased by a chaplain assigned by the morgue. Therefore, it is important for the morgue to know the religious affiliation of the deceased, or personal requests from the next of kin, at the time of admission. Maximum Period of Storage Before Burial Zambian law does not specify a period in which burial of remains must take place, regardless of embalmment. Zambia is a semi-tropical country, and it is customary that burials take place soon after death. The normally acceptable time period is three days, after which additional storage charges may accrue. Embalming Embalming facilities exist at Ambassador St. Ann Funeral Home and FSG Ltd. (Ideal Funeral Home). The standards are acceptable but vary depending on the condition of the body at the time of preparation. Facilities for holding embalmed remains are available. Caskets and Containers Caskets and containers are available locally and meet international shipping requirements. The remains are placed in a hermetically sealed zinc casket, which is then placed in a wooden crate prior to air shipment. Cremation Following cremation, the remains are placed in an urn which is enclosed in a container for return to the United States as air cargo. -

Processing of Konkola Copper Concentrates and Chingola Refractory Ore
Processing of Konkola copper concen- trates and Chingola refractory ore in a fully integrated hydrometallurgical pilot plant circuit by R.M. Whyte*, N. Schoeman†, and K.G. Bowes‡ terized by the size and quality of its copper and cobalt deposits. The Nampundwe mine Synopsis situated south-west of Lusaka is of strategic importance to the metallurgical operations on The Konkola Deep Mining Project (KDMP) seeks to exploit the deep the copperbelt, supplying a high sulphur pyrite level potential of the Konkola Deeps orebody, producing high grade concentrate to the cobalt roast-leach- copper concentrates for treatment at the Nkana Smelter and electrowinning (RLE) plants, and to the copper Refinery. smelters to make up for a sulphur deficit in the As an alternative to smelting, Konkola Copper Mines (KCM) has smelter feed. examined a processing route involving pressure leaching of the The Konkola mine commenced production Konkola concentrates at Nchanga, with the leach liquor being in 1957. The mine is one of the wettest in the treated by solvent extraction and electrowinning to produce high world, with a total of 300 000 cubic metres of purity copper cathode. The solvent extraction circuit would be operated to achieve a high ‘delta’ copper, thereby providing a water being pumped daily from the strong acid raffinate which can be used in the existing Tailings underground mine. Current mine production Leach Plant (TLP) to treat Nchanga oxide copper materials. from Konkola’s Kililabombwe orebody The main focus of the hydrometallurgical option is to capitalize amounts to approximately 2 million ton per upon the synergy that exists between the treatment of sulphide year, and this ore is treated through a conven- copper concentrates (acid producing) and oxide copper ores (acid tional crushing, milling and flotation plant. -
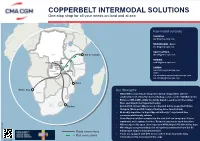
Copperbelt Intermodal Solutions
t s a o C t es W ica er m A th u o S m o r F COPPERBELT INTERMODAL SOLUTIONS / One-stop shop for all your needs on land and at sea o T Intermodal contacts TANZANIA [email protected] MOZAMBIQUE - Beira [email protected] TANZANIA SOUTH AFRICA DRC Dar Es Salaam [email protected] NAMIBIA Kambove Kolwezi Likasi [email protected] Lubumbashi Kitwe ZAMBIA Ndola MOZAMBIQUE [email protected] ZAMBIA Kapiri Mposhi Lusaka Sales [email protected] [email protected] Beira Walvis Bay Our Strengths NAMIBIA • CMA CGM is extending its footprint in Africa’s Copperbelt, with the establishment of a New Rail Carrier Haulage service on the TAZARA rail line • Enhance CMA CGM’s ability to provide logistics services for the mining SOUTH firms operating in the Copperbelt region AFRICA Durban • Bonded ICD at Kapiri Mposhi used a dispatch hub to CopperBelt (Kitwe, Chingola, Ndola and DR Congo). Final leg to be done by truck • Bi-weekly departures to Kapiri Mposhi with only 7 days transit time, environmental-friendly solution • Cost effective solution compared to the road (rail can carry up to 30 tons maximum), well organized service. Reduced exposure to truck detention • ICD storage facility upon client request at MCCL/Kapiri ICD with 30 free days • Will mitigate congestion/delay risk and optimize transit time from Dar Es Road connections Salaam port. Improved turnaround times • Trains are equipped with GPS devices which helps to provide daily Rail connections information of the movement of the cargo www.cma-cgm.com June 2020. -

Registered Voters by Gender and Constituency
REGISTERED VOTERS BY GENDER AND CONSTITUENCY % OF % OF SUB % OF PROVINCIAL CONSTITUENCY NAME MALES MALES FEMALES FEMALES TOTAL TOTAL KATUBA 25,040 46.6% 28,746 53.4% 53,786 8.1% KEEMBE 23,580 48.1% 25,453 51.9% 49,033 7.4% CHISAMBA 19,289 47.5% 21,343 52.5% 40,632 6.1% CHITAMBO 11,720 44.1% 14,879 55.9% 26,599 4.0% ITEZH-ITEZHI 18,713 47.2% 20,928 52.8% 39,641 5.9% BWACHA 24,749 48.1% 26,707 51.9% 51,456 7.7% KABWE CENTRAL 31,504 47.4% 34,993 52.6% 66,497 10.0% KAPIRI MPOSHI 41,947 46.7% 47,905 53.3% 89,852 13.5% MKUSHI SOUTH 10,797 47.3% 12,017 52.7% 22,814 3.4% MKUSHI NORTH 26,983 49.5% 27,504 50.5% 54,487 8.2% MUMBWA 23,494 47.9% 25,545 52.1% 49,039 7.4% NANGOMA 12,487 47.4% 13,864 52.6% 26,351 4.0% LUFUBU 5,491 48.1% 5,920 51.9% 11,411 1.7% MUCHINGA 10,072 49.7% 10,200 50.3% 20,272 3.0% SERENJE 14,415 48.5% 15,313 51.5% 29,728 4.5% MWEMBEZHI 16,756 47.9% 18,246 52.1% 35,002 5.3% 317,037 47.6% 349,563 52.4% 666,600 100.0% % OF % OF SUB % OF PROVINCIAL CONSTITUENCY NAME MALES MALES FEMALES FEMALES TOTAL TOTAL CHILILABOMBWE 28,058 51.1% 26,835 48.9% 54,893 5.4% CHINGOLA 34,695 49.7% 35,098 50.3% 69,793 6.8% NCHANGA 23,622 50.0% 23,654 50.0% 47,276 4.6% KALULUSHI 32,683 50.1% 32,614 49.9% 65,297 6.4% CHIMWEMWE 29,370 48.7% 30,953 51.3% 60,323 5.9% KAMFINSA 24,282 51.1% 23,214 48.9% 47,496 4.6% KWACHA 31,637 49.3% 32,508 50.7% 64,145 6.3% NKANA 27,595 51.9% 25,562 48.1% 53,157 5.2% WUSAKILE 23,206 50.5% 22,787 49.5% 45,993 4.5% LUANSHYA 26,658 49.5% 27,225 50.5% 53,883 5.3% ROAN 15,921 50.1% 15,880 49.9% 31,801 3.1% LUFWANYAMA 18,023 50.2% -

Members of the Northern Rhodesia Legislative Council and National Assembly of Zambia, 1924-2021
NATIONAL ASSEMBLY OF ZAMBIA Parliament Buildings P.O Box 31299 Lusaka www.parliament.gov.zm MEMBERS OF THE NORTHERN RHODESIA LEGISLATIVE COUNCIL AND NATIONAL ASSEMBLY OF ZAMBIA, 1924-2021 FIRST EDITION, 2021 TABLE OF CONTENTS FOREWORD ................................................................................................................................................ 3 PREFACE ..................................................................................................................................................... 4 ACKNOWLEDGEMENTS .......................................................................................................................... 5 ABBREVIATIONS ...................................................................................................................................... 7 INTRODUCTION ........................................................................................................................................ 9 PART A: MEMBERS OF THE LEGISLATIVE COUNCIL, 1924 - 1964 ............................................... 10 PRIME MINISTERS OF THE FEDERATION OF RHODESIA .......................................................... 12 GOVERNORS OF NORTHERN RHODESIA AND PRESIDING OFFICERS OF THE LEGISTRATIVE COUNCIL (LEGICO) ............................................................................................... 13 SPEAKERS OF THE LEGISTRATIVE COUNCIL (LEGICO) - 1948 TO 1964 ................................. 16 DEPUTY SPEAKERS OF THE LEGICO 1948 TO 1964 .................................................................... -

Zambia Country Profile Report
TABLE OF CONTENTS LIST OF FIGURES ................................................................................................................ i LIST OF TABLES .................................................................................................................. i COUNTRY FACT SHEET ...................................................................................................... i LIST OF ACRONYMS .......................................................................................................... ii EXECUTIVE SUMMARY ....................................................................................................... i 1. INTRODUCTION AND BACKGROUND ........................................................................ 1 1.1 Introduction ............................................................................................................................ 1 1.2 Background ........................................................................................................................... 2 2. OVERVIEW OF ZAMBIA ............................................................................................... 4 3. ECONOMIC OUTLOOK ................................................................................................ 7 3.1 Economy ..................................................................................................................................... 7 3.2 Economic Structure .................................................................................................................. -

Zambia Non-Motorised Transport Strategy
Zambia Non-Motorised Transport Strategy Ministry of Transport and Communications United Nations Environment Programme Institute for Transportation and Development Policy July 2019 Contents 1. Introduction ...................................................................................................................................... 3 2. Emerging urban mobility challenges ................................................................................................ 3 3. Assessment of walking & cycling environment ............................................................................... 5 3.1 Footpaths ............................................................................................................................... 5 3.2 Cycle facilities ....................................................................................................................... 7 3.3 Pedestrian crossings ............................................................................................................... 7 3.4 Parking management ............................................................................................................. 8 3.5 Street lighting ........................................................................................................................ 9 3.6 Storm water management .................................................................................................... 10 3.7 Building design ................................................................................................................... -

Konkola Newsnews •• Juneaugust 2017 2017 • Issue • Issue N°73 N°72 P.1 Konkola News Issue N°72 • August 2017 •
KonkolaKonkola NewsNews •• JuneAugust 2017 2017 • Issue • Issue N°73 N°72 p.1 Konkola News Issue N°72 • August 2017 • www.kcm.co.zm Content p.3 p.6 p.18 p.28 Pongamia trees Women in Clean water to Rangers, Blades planted to engineering toast 8,000 Chingola youth stars join rehabilitate mine on their day residents international site p.14 p.22 football academy p.5 Memoirs of Anecdote of Kitwe Vedanta hosts longest serving Trades School and third Sustainable underground the Twins! Development Day miner Konkola News • August 2017 • Issue N°72 p.2 Message from the CEO Dear Colleagues We have made good progress on our journey towards establishing KCM as the Pride of Zambia. ‘12-5 Make-it-Happen’ Through our ‘12-5’ production campaign, we are restoring pride in this great company and growing our business sustainably. With the same focus, determination and teamwork, I am confident that we will achieve the target of 12,500 tonnes of integrated copper production. This will be a significant milestone and a cause for celebration. KCM Chief Executive Officer Steven Din The copper price is picking up steadily and, together with the improvements we are making to our operations, I am optimistic that we can deliver strong results for the full year. Having said this, there’s still a lot of work that needs to be done on the ground and there’s no room for complacency. Safety Safety is the cornerstone of our business. The Chingilila safety campaign is bearing fruit and our safety statistics are trending to acceptable levels. -

Chingola District
CHINGOLA DISTRICT INVESTMENT PROFILE Compiled by: Director Planning Unit 1 Contents 1.0 Overview ................................................................................................................. 4 1.1 Climate ................................................................................................................ 4 1.2 Demography and Socio-Economic Profile................................................................. 6 1.3 Transportation in the District ...................................................................................... 7 2.0 INVESTMENT OPPORTUNITIES IN THE DISTRICT ........................................................... 7 2.1 Infrastructure (Property Development) Sector ............................................................. 9 2.2 Manufacturing Industry - Mineral Processing in Chingola. ............................................12 2.3 Agriculture Sector ....................................................................................................12 2.3.1 Fish Farming ......................................................................................................12 2.3.2 Investing in Fish Farming ....................................................................................13 2.3.3 Crop Production .................................................................................................13 2.3.4. Investing in Crop Production ..............................................................................14 2.3.5 Livestock Production ...........................................................................................14 -

The General Public Is Hereby Advised That Any Person Who Constructs
PUBLIC NOTICE LIST OF LICENSED ELECTRONIC COMMUNICATION OPERATORS The general public is hereby advised that any person who constructs, owns or makes available an electronic communications network, provides a network service or provides one or more electronic communication services without a valid licence is guilty of an offence, as provided for under the Information and Communications Technology Act of No. 15 2009. Accordingly, this serves to notify members of the general public that entities listed below are duly licensed to establish or provide or offer communication service or indeed to connect apparatus to an electronic communication system or work any such apparatus for the purpose of a telecommunication system;- Licence Register as at June 30, 2020 NETWORK (SERVICE & FACILITIES) AND SERVICE CATEROGY A LICENCES # LICENCE NUMBER NAME OF LICENSEE LICENCE TYPE MARKET SEGMENT 1. ZICTA/ICT/ECN/ECS 0001 Zambia Telecommunications Company Limited Electronic International P O Box 37000 Communication Network LUSAKA Electronic National Communication Service 2. ZICTA/ICT/ECN/ECS 0002 Airtel Networks Zambia Plc Electronic International Airtel House, Stand 2375 Communication Network Cnr East Road & Addis Ababa Drive P O Box Electronic 320001, National Communication Service LUSAKA 3. ZICTA/ICT/ECN/ECS 0003 MTN Zambia Limited Electronic Maanu Centre,Stand No. 4647 International Communication Network Beit Road, Addis Ababa Roundabout P.O. Box 35464 Electronic National LUSAKA Communication Services 4. ZICTA/ICT/ECN 0004 Zambia Electricity Supply Corporation Limited Stand No. 6949 Electronic Great East Road National Communication Network P O Box 33304 LUSAKA 5. ZICTA/ICT/ECN/ECS 0005 Liquid Telecommunications Zambia Limited Electronic National Eluda II, 3rd Floor Communication Network Stand No.