Chlamydia Trachomatis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chlamydia Trachomatis Infection Is Driven by Nonprotective Immune Cells That Are Distinct from Protective Populations
Pathology after Chlamydia trachomatis infection is driven by nonprotective immune cells that are distinct from protective populations Rebeccah S. Lijeka,b,1, Jennifer D. Helblea, Andrew J. Olivea,c, Kyra W. Seigerb, and Michael N. Starnbacha,1 aDepartment of Microbiology and Immunobiology, Harvard Medical School, Boston, MA 02115; bDepartment of Biological Sciences, Mount Holyoke College, South Hadley, MA 01075; and cDepartment of Microbiology and Physiological Systems, University of Massachusetts Medical School, Worcester, MA 01605 Edited by Rafi Ahmed, Emory University, Atlanta, GA, and approved December 27, 2017 (received for review June 23, 2017) Infection with Chlamydia trachomatis drives severe mucosal immu- sequence identity, Chlamydia muridarum, the extent to which the nopathology; however, the immune responses that are required for molecular pathogenesis of C. muridarum represents that of Ct is mediating pathology vs. protection are not well understood. Here, unknown (6). Ct serovar L2 (Ct L2) is capable of infecting the we employed a mouse model to identify immune responses re- mouse upper genital tract when inoculated across the cervix into quired for C. trachomatis-induced upper genital tract pathology the uterus (7, 8) but it does not induce robust immunopathology. and to determine whether these responses are also required for This is consistent with the human disease phenotype caused by Ct L2, bacterial clearance. In mice as in humans, immunopathology was which disseminates to the lymph nodes causing lymphogranuloma characterized by extravasation of leukocytes into the upper genital venereum (LGV) and is not a major cause of mucosal immunopa- thology in the female upper genital tract (uterus and ovaries). tract that occluded luminal spaces in the uterus and ovaries. -

Leptospirosis: a Waterborne Zoonotic Disease of Global Importance
August 2006 volume 22 number 08 Leptospirosis: A waterborne zoonotic disease of global importance INTRODUCTION syndrome has two phases: a septicemic and an immune phase (Levett, 2005). Leptospirosis is considered one of the most common zoonotic diseases It is in the immune phase that organ-specific damage and more severe illness globally. In the United States, outbreaks are increasingly being reported is seen. See text box for more information on the two phases. The typical among those participating in recreational water activities (Centers for Disease presenting signs of leptospirosis in humans are fever, headache, chills, con- Control and Prevention [CDC], 1996, 1998, and 2001) and sporadic cases are junctival suffusion, and myalgia (particularly in calf and lumbar areas) often underdiagnosed. With the onset of warm temperatures, increased (Heymann, 2004). Less common signs include a biphasic fever, meningitis, outdoor activities, and travel, Georgia may expect to see more leptospirosis photosensitivity, rash, and hepatic or renal failure. cases. DIAGNOSIS OF LEPTOSPIROSIS Leptospirosis is a zoonosis caused by infection with the bacterium Leptospira Detecting serum antibodies against leptospira interrogans. The disease occurs worldwide, but it is most common in temper- • Microscopic Agglutination Titers (MAT) ate regions in the late summer and early fall and in tropical regions during o Paired serum samples which show a four-fold rise in rainy seasons. It is not surprising that Hawaii has the highest incidence of titer confirm the diagnosis; a single high titer in a per- leptospirosis in the United States (Levett, 2005). The reservoir of pathogenic son clinically suspected to have leptospirosis is highly leptospires is the renal tubules of wild and domestic animals. -

Gonorrhea, Chlamydia, and Syphilis
2019 GONORRHEA, CHLAMYDIA, AND SYPHILIS AND CHLAMYDIA, GONORRHEA, Dedication TAG would like to thank the National Coalition of STD Directors for funding and input on the report. THE PIPELINE REPORT Pipeline for Gonorrhea, Chlamydia, and Syphilis By Jeremiah Johnson Introduction The current toolbox for addressing gonorrhea, chlamydia, and syphilis is inadequate. At a time where all three epidemics are dramatically expanding in locations all around the globe, including record-breaking rates of new infections in the United States, stakeholders must make do with old tools, inadequate systems for addressing sexual health, and a sparse research pipeline of new treatment, prevention, and diagnostic options. Lack of investment in sexual health research has left the field with inadequate prevention options, and limited access to infrastructure for testing and treatment have allowed sexually transmitted infections (STIs) to flourish. The consequences of this underinvestment are large: according to the World Health Organization (WHO), in 2012 there were an estimated 357 million new infections (roughly 1 million per day) of the four curable STIs: gonorrhea, chlamydia, syphilis, and trichomoniasis.1 In the United States, the three reportable STIs that are the focus of this report—gonorrhea, chlamydia, and syphilis—are growing at record paces. In 2017, a total of 30,644 cases of primary and secondary (P&S) syphilis—the most infectious stages of the disease—were reported in the United States. Since reaching a historic low in 2000 and 2001, the rate of P&S syphilis has increased almost every year, increasing 10.5% during 2016–2017. Also in 2017, 555,608 cases of gonorrhea were reported to the U.S. -
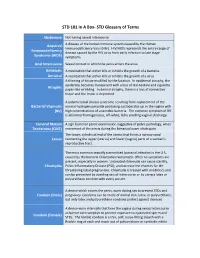
STD Glossary of Terms
STD 101 In A Box- STD Glossary of Terms Abstinence Not having sexual intercourse Acquired A disease of the human immune system caused by the Human Immunodeficiency Virus (HIV). HIV/AIDS represents the entire range of Immunodeficiency disease caused by the HIV virus from early infection to late stage Syndrome (AIDS) symptoms. Anal Intercourse Sexual contact in which the penis enters the anus. Antibiotic A medication that either kills or inhibits the growth of a bacteria. Antiviral A medication that either kills or inhibits the growth of a virus. A thinning of tissue modified by the location. In epidermal atrophy, the epidermis becomes transparent with a loss of skin texture and cigarette Atrophic paper-like wrinkling. In dermal atrophy, there is a loss of connective tissue and the lesion is depressed. A polymicrobial clinical syndrome resulting from replacement of the Bacterial Vaginosis normal hydrogen peroxide producing Lactobacillus sp. in the vagina with (BV) high concentrations of anaerobic bacteria. The common symptom of BV is abnormal homogeneous, off-white, fishy smelling vaginal discharge. Cervical Motion A sign found on pelvic examination suggestive of pelvic pathology; when Tenderness (CMT) movement of the cervix during the bimanual exam elicits pain. The lower, cylindrical end of the uterus that forms a narrow canal Cervix connecting the upper (uterus) and lower (vagina) parts of a woman's reproductive tract. The most common sexually transmitted bacterial infection in the U.S., caused by the bacteria Chlamydia trachomatis. Often no symptoms are present, especially in women. Untreated chlamydia can cause sterility, Chlamydia Pelvic Inflammatory Disease (PID), and increase the chances for life- threatening tubal pregnancies. -

Chlamydia Trachomatis and Chlamydia Pneumoniae Interaction with the Host: Latest Advances and Future Prospective
microorganisms Review Chlamydia trachomatis and Chlamydia pneumoniae Interaction with the Host: Latest Advances and Future Prospective Marisa Di Pietro 1,* , Simone Filardo 1 , Silvio Romano 2 and Rosa Sessa 1 1 Department of Public Health and Infectious Diseases, Section of Microbiology, University of Rome “Sapienza”, 00185 Rome, Italy; simone.fi[email protected] (S.F.); [email protected] (R.S.) 2 Cardiology, Department of Life, Health and Environmental Sciences, University of L’Aquila, 67100 L’Aquila, Italy; [email protected] * Correspondence: [email protected] Received: 15 April 2019; Accepted: 14 May 2019; Published: 16 May 2019 Abstract: Research in Chlamydia trachomatis and Chlamydia pneumoniae has gained new traction due to recent advances in molecular biology, namely the widespread use of the metagenomic analysis and the development of a stable genomic transformation system, resulting in a better understanding of Chlamydia pathogenesis. C. trachomatis, the leading cause of bacterial sexually transmitted diseases, is responsible of cervicitis and urethritis, and C. pneumoniae, a widespread respiratory pathogen, has long been associated with several chronic inflammatory diseases with great impact on public health. The present review summarizes the current evidence regarding the complex interplay between C. trachomatis and host defense factors in the genital micro-environment as well as the key findings in chronic inflammatory diseases associated to C. pneumoniae. Keywords: Chlamydia trachomatis; Chlamydia pneumoniae; host-pathogen interaction 1. Introduction Currently, there is a renewed research interest in Chlamydiae that cause a broad spectrum of pathologies of varying severity in human, mainly Chlamydia trachomatis and Chlamydia pneumoniae [1,2]. Advances in molecular biology and, in particular, the recent advent of metagenomic analysis as well as the development of a stable genomic transformation system in Chlamydiae have significantly contributed to expanding our understanding of Chlamydia pathogenesis [3–5]. -

Chlamydial Genital Infection(Chlamydia Trachomatis)
Chlamydial Genital Infection (Chlamydia trachomatis) February 2003 1) THE DISEASE AND ITS EPIDEMIOLOGY A. Etiologic Agent Chlamydial genital infection (CGI) is caused by the obligate, intracellular bacterium Chlamydia trachomatis immunotypes D through K. B. Clinical Description and Laboratory Diagnosis A sexually transmitted genital infection that manifests in males primarily as urethritis and in females as mucopurulent cervicitis. Clinical manifestations are difficult to distinguish from gonorrhea. Males may present with a mucopurulent discharges of scanty to moderate quantity, urethral itching and dysuria. Asymptomatic infection may be found in 1%-25% of sexually active men. Possible complications include epididymitis, infertility and Reiter syndrome. Anorectal intercourse may result in chlamydial proctitis. Women frequently present with a mucopurulent endocervical discharge including edema, erythema and easily induced endocervical bleeding. However, most women with endocervical or urethral infections are asymptomatic. Possible complications include salpingitis with subsequent risk of infertility and ectopic pregnancy. Asymptomatic chronic infections of the endometrium and fallopian tubes may lead to the same outcomes. Less frequent manifestations include bartholinitis, urethral syndrome with dysuria and pyuria, perihepatitis (Fitz-Hugh-Curtis syndrome), and proctitis. Infection during pregnancy may result in premature rupture of membranes and preterm delivery and conjunctival and pneumonic infection of the newborn. Laboratory diagnosis is based upon the identification of Chlamydia in intraurethral or endocervical smear by direct immunofluorescence test, enzyme immunoassay, DNA probe, and nucleic acid amplification test (NAAT) or cell culture. NAAT can be used with urine specimens. C. Vectors and Reservoirs Humans. D. Modes of Transmission By sexual contact and through perinatal exposure to the mother’s infected cervix. -

Chlamydia, Gonorrhoea, Trichomoniasis and Syphilis
Research Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016 Jane Rowley,a Stephen Vander Hoorn,b Eline Korenromp,c Nicola Low,d Magnus Unemo,e Laith J Abu- Raddad,f R Matthew Chico,g Alex Smolak,f Lori Newman,h Sami Gottlieb,a Soe Soe Thwin,a Nathalie Brouteta & Melanie M Taylora Objective To generate estimates of the global prevalence and incidence of urogenital infection with chlamydia, gonorrhoea, trichomoniasis and syphilis in women and men, aged 15–49 years, in 2016. Methods For chlamydia, gonorrhoea and trichomoniasis, we systematically searched for studies conducted between 2009 and 2016 reporting prevalence. We also consulted regional experts. To generate estimates, we used Bayesian meta-analysis. For syphilis, we aggregated the national estimates generated by using Spectrum-STI. Findings For chlamydia, gonorrhoea and/or trichomoniasis, 130 studies were eligible. For syphilis, the Spectrum-STI database contained 978 data points for the same period. The 2016 global prevalence estimates in women were: chlamydia 3.8% (95% uncertainty interval, UI: 3.3–4.5); gonorrhoea 0.9% (95% UI: 0.7–1.1); trichomoniasis 5.3% (95% UI:4.0–7.2); and syphilis 0.5% (95% UI: 0.4–0.6). In men prevalence estimates were: chlamydia 2.7% (95% UI: 1.9–3.7); gonorrhoea 0.7% (95% UI: 0.5–1.1); trichomoniasis 0.6% (95% UI: 0.4–0.9); and syphilis 0.5% (95% UI: 0.4–0.6). Total estimated incident cases were 376.4 million: 127.2 million (95% UI: 95.1–165.9 million) chlamydia cases; 86.9 million (95% UI: 58.6–123.4 million) gonorrhoea cases; 156.0 million (95% UI: 103.4–231.2 million) trichomoniasis cases; and 6.3 million (95% UI: 5.5–7.1 million) syphilis cases. -

Evaluation and Determination of the Sensitivity and Specificity of a Treponema Pallidum Dried Blood Spot Method for Serologic Diagnosis of Syphilis
Georgia State University ScholarWorks @ Georgia State University Public Health Theses School of Public Health Fall 12-20-2012 Evaluation and Determination of the Sensitivity and Specificity of a Treponema Pallidum Dried Blood Spot Method for Serologic Diagnosis of Syphilis David K. Turgeon Follow this and additional works at: https://scholarworks.gsu.edu/iph_theses Recommended Citation Turgeon, David K., "Evaluation and Determination of the Sensitivity and Specificity of a rT eponema Pallidum Dried Blood Spot Method for Serologic Diagnosis of Syphilis." Thesis, Georgia State University, 2012. https://scholarworks.gsu.edu/iph_theses/239 This Thesis is brought to you for free and open access by the School of Public Health at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Public Health Theses by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. Institute of Public Health Public Health Thesis Georgia State University Year 2012 EVALUATION AND DETERMINATION OF THE SENSITIVITY AND SPECIFICITY OF A Treponema pallidum DRIED BLOOD SPOT METHOD FOR SEROLOGIC DIAGNOSIS OF SYPHILIS David K. Turgeon Georgia State University, [email protected] ii ABSTRACT EVALUATION AND DETERMINATION OF THE SENSITIVITY AND SPECIFICITY OF A Treponema pallidum DRIED BLOOD SPOT (DBS) METHOD FOR SEROLOGIC DIAGNOSIS OF SYPHILIS Background: Syphilis is a sexually transmitted infection (STI) caused by Treponema pallidum subspecies pallidum. Syphilis is known as the “great imitator" due to the similarity of clinical signs and symptoms to other infectious diseases. The primary diagnosis of syphilis relies on clinical findings, including the examination of treponemal lesions, and/or serologic tests. -

Detection of Kaposi's Sarcoma–Associated Herpesvirus in Oral
1785 CONCISE COMMUNICATION Detection of Kaposi's Sarcoma±Associated Herpesvirus in Oral and Genital Secretions of Zimbabwean Women Thomas M. Lampinen,1,3 Shalini Kulasingam,1 1Departments of Epidemiology and 2Pathobiology and 3Center for AIDS Juno Min,4 Margaret Borok,6 Lovemore Gwanzura,7 and STD, University of Washington, Seattle; 4Department of Medicine 5 Downloaded from https://academic.oup.com/jid/article/181/5/1785/2191825 by guest on 29 September 2021 4 8 9 and Center for AIDS Research, Stanford University, Stanford, Julie Lamb, Kassam Mahomed, Godfrey B. Woelk, 6 7 2 2 California; Departments of Medicine, Medical Laboratory Sciences, Kurt B. Strand, Marnix L. Bosch, 8Obstetrics and Gynecology, and 9Community Medicine, 10 10,11 Daniel C. Edelman, Niel T. Constantine, University of Zimbabwe, Harare; 10Department of Pathology, David Katzenstein,4,5 and Michelle A. Williams1 University of Maryland and 11Institute of Human Virology, Baltimore, Maryland Kaposi's sarcoma±associated herpesvirus (KSHV) in oral and genital secretions of women may be involved in horizontal and vertical transmission in endemic regions. Nested polymerase chain reaction assays were used to detect KSHV DNA sequences in one-third of oral, vaginal, and cervical specimens and in 42% of peripheral blood mononuclear cell (PBMC) specimens collected from 41 women infected with human immunode®ciency virus type 1 who had Ka- posi's sarcoma (KS). KSHV DNA was not detected in specimens from 100 women without KS, 9 of whom were seropositive for KSHV. A positive association was observed between KSHV DNA detection in oral and genital mucosa, neither of which was associated with KSHV DNA detection in PBMC. -

Laboratory Diagnosis of Sexually Transmitted Infections, Including Human Immunodeficiency Virus
Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus human immunodeficiency including Laboratory transmitted infections, diagnosis of sexually Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus Editor-in-Chief Magnus Unemo Editors Ronald Ballard, Catherine Ison, David Lewis, Francis Ndowa, Rosanna Peeling For more information, please contact: Department of Reproductive Health and Research World Health Organization Avenue Appia 20, CH-1211 Geneva 27, Switzerland ISBN 978 92 4 150584 0 Fax: +41 22 791 4171 E-mail: [email protected] www.who.int/reproductivehealth 7892419 505840 WHO_STI-HIV_lab_manual_cover_final_spread_revised.indd 1 02/07/2013 14:45 Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus Editor-in-Chief Magnus Unemo Editors Ronald Ballard Catherine Ison David Lewis Francis Ndowa Rosanna Peeling WHO Library Cataloguing-in-Publication Data Laboratory diagnosis of sexually transmitted infections, including human immunodeficiency virus / edited by Magnus Unemo … [et al]. 1.Sexually transmitted diseases – diagnosis. 2.HIV infections – diagnosis. 3.Diagnostic techniques and procedures. 4.Laboratories. I.Unemo, Magnus. II.Ballard, Ronald. III.Ison, Catherine. IV.Lewis, David. V.Ndowa, Francis. VI.Peeling, Rosanna. VII.World Health Organization. ISBN 978 92 4 150584 0 (NLM classification: WC 503.1) © World Health Organization 2013 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for non-commercial distribution – should be addressed to WHO Press through the WHO web site (www.who.int/about/licensing/copyright_form/en/index.html). -

Chlamydia Trachomatis Strains and Virulence: Rethinking Links to Infection Prevalence and Disease Severity
SUPPLEMENT ARTICLE Chlamydia trachomatis Strains and Virulence: Rethinking Links to Infection Prevalence and Disease Severity Gerald I. Byrne Department of Molecular Sciences, University of Tennessee Health Science Center, Memphis An unanswered question concerning prevalence and disease severity of Chlamydia trachomatis genital infection is whether more prevalent strains or strains more likely to cause serious disease complications are causally associated with specific virulence attributes. The major method for distinguishing chlamydial strains is based on differences in the major outer membrane protein (MOMP). A subset of MOMP serovars (D and E serovars) are easily the most prevalent strains identified worldwide, but MOMP serovar and genovar analyses have not yielded consistent strain-dependent virulence distinctions. Expansion of the definitions of chlamydial strains beyond the MOMP paradigm are needed to better understand virulence properties for this pathogen and how these properties reflect disease severity. Substantive genetic and phenotypic differences have emerged for the 2 major C. trachomatis pathobiotypes associated with either trachoma or sexually transmitted diseases, but differences within the sexually transmitted disease group have not yielded reliable disease severity attributes. A number of candidate virulence factors have been identified, including the polymorphic outer membrane autotransporter family of proteins, the putative large cytotoxin, type III secretion effectors, stress response proteins, and proteins or other regulatory factors produced by the cryptic plasmid. Continued work on development of a chlamydial gene transfer system and application of genomic approaches to large collections of clinical isolates will be required to associate key chlamydial virulence factors with prevalence and disease severity in a definitive way. Identification and sorting of different Chlamydia tra- ease in women or other serious complications of chla- chomatis genital tract isolates have been central to ep- mydial genital tract infection [1]. -

Chlamydia Trachomatis
CHLAMYDIA TRACHOMATIS REPORTING INFORMATION • Class B: Report the case, suspected case and/or a positive laboratory result to the local public health department where the patient resides by the close of the next business day. If patient residence is unknown, report to the local public health department in which the reporting health care provider or laboratory is located. • Health care providers and laboratories report using the following form(s) and/or mechanism: Ohio Confidential Reportable Disease form (HEA 3334, rev. 5/2014), Positive Laboratory Findings for Reportable Disease form (HEA 3333, rev. 8/2005), Ohio Disease Reporting System (ODRS), electronic laboratory reporting (ELR), or telephone. • Local public health departments report the case, suspected case and/or a positive laboratory result to the Ohio Department of Health (ODH) via ODRS by the end of the next business day. • Key fields for ODRS reporting include: for laboratory - date collected, test name, and result; for clinical - treatment name, dose and start date. AGENT Chlamydia trachomatis (CT) is an obligate intracellular organism that is Gram-negative, ovoid in shape, and nonmobile. Most genital isolates belong to immunotypes D through K. CASE DEFINITION Clinical Description Infection with Chlamydia trachomatis may result in urethritis, epididymitis, cervicitis, acute salpingitis, or other syndromes when sexually transmitted; however, the infection is often asymptomatic in women. Perinatal infections may result in inclusion conjunctivitis and pneumonia in newborns. Other syndromes caused by C. trachomatis include lymphogranuloma venereum and trachoma. Laboratory Criteria for Diagnosis • Isolation of C. trachomatis by culture OR • Demonstration of C. trachomatis in a clinical specimen by detection of antigen or nucleic acid.