10 Functional Groups of Organic Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Names for Selected Aromatic Groups
More Nomenclature: Common Names for Selected Aromatic Groups Phenyl group = or Ph = C6H5 = Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings. Benzyl = Bn = It has a -CH2- (methylene) group attached to the benzene ring. This group can be used to name particular compounds, such as the one shown below. This compound has chlorine attached to a benzyl group, therefore it is called benzyl chloride. Benzoyl = Bz = . This is different from benzyl group (there is an extra “o” in the name). It has a carbonyl attached to the benzene ring instead of a methylene group. For example, is named benzoyl chloride. Therefore, it is sometimes helpful to recognize a common structure in order to name a compound. Example: Nomenclature: 3-phenylpentane Example: This is Amaize. It is used to enhance the yield of corn production. The systematic name for this compound is 2,4-dinitro-6-(1-methylpropyl)phenol. Polynuclear Aromatic Compounds Aromatic rings can fuse together to form polynuclear aromatic compounds. Example: It is two benzene rings fused together, and it is aromatic. The electrons are delocalized in both rings (think about all of its resonance form). Example: This compound is also aromatic, including the ring in the middle. All carbons are sp2 hybridized and the electron density is shared across all 5 rings. Example: DDT is an insecticide and helped to wipe out malaria in many parts of the world. Consequently, the person who discovered it (Muller) won the Nobel Prize in 1942. The systematic name for this compound is 1,1,1-trichloro-2,2-bis-(4-chlorophenyl)ethane. -

Fischer Carbene Complexes in Organic Synthesis Ke Chen 1/31/2007
Baran Group Meeting Fischer Carbene Complexes in Organic Synthesis Ke Chen 1/31/2007 Ernst Otto Fischer (1918 - ) Other Types of Stabilized Carbenes: German inorganic chemist. Born in Munich Schrock carbene, named after Richard R. Schrock, is nucleophilic on November 10, 1918. Studied at Munich at the carbene carbon atom in an unpaired triplet state. Technical University and spent his career there. Became director of the inorganic Comparision of Fisher Carbene and Schrock carbene: chemistry institute in 1964. In the 1960s, discovered a metal alkylidene and alkylidyne complexes, referred to as Fischer carbenes and Fischer carbynes. Shared the Nobel Prize in Chemistry with Geoffery Wilkinson in 1973, for the pioneering work on the chemistry of organometallic compounds. Schrock carbenes are found with: Representatives: high oxidation states Isolation of first transition-metal carbene complex: CH early transition metals Ti(IV), Ta(V) 2 non pi-acceptor ligands Cp2Ta CH N Me LiMe Me 2 2 non pi-donor substituents CH3 (CO) W CO (CO)5W 5 (CO)5W A.B. Charette J. Am. Chem. Soc. 2001, 123, 11829. OMe O E. O. Fischer, A. Maasbol, Angew. Chem. Int. Ed., 1964, 3, 580. Persistent carbenes, isolated as a crystalline solid by Anthony J. Arduengo in 1991, can exist in the singlet state or the triplet state. Representative Fischer Carbenes: W(CO) Cr(CO) 5 5 Fe(CO)4 Mn(CO)2(MeCp) Co(CO)3SnPh3 Me OMe Ph Ph Ph NEt2 Ph OTiCp2Cl Me OMe Foiled carbenes were defined as "systems where stabilization is Fischer carbenes are found with : obtained by the inception of the facile reaction which is foiled by the impossibility of attaining the final product geometry". -

23.5 Basicity and Acidity of Amines
23_BRCLoudon_pgs5-0.qxd 12/8/08 1:22 PM Page 1122 1122 CHAPTER 23 • THE CHEMISTRY OF AMINES alkylamines, this resonance occurs at rather small chemical shift—typically around d 1. In aromatic amines, this resonance is at greater chemical shift, as in the second of the preceding examples. Like the OH protons of alcohols, phenols, and carboxylic acids, the NH protons of amines under most conditions undergo rapid exchange (Secs. 13.6 and 13.7D). For this reason, split- ting between the amine N H and adjacent C H groups is usually not observed. Thus, in the NMR spectrum of diethylamine,L the N H resonanceL is a singlet rather than the triplet ex- pected from splitting by the adjacent CHL2 protons. In some amine samples, the N H resonance is broadened and, like the OL H protonL of alcohols, it can be obliterated fromL the spectrum by exchange with D2O (the “DL2O shake,” p. 611). The characteristic 13C NMR absorptions of amines are those of the a-carbons—the carbons attached directly to the nitrogen. These absorptions occur in the d 30–50 chemical-shift range. As expected from the relative electronegativities of oxygen and nitrogen, these shifts are somewhat less than the a-carbon shifts of ethers. PROBLEMS 23.4 Identify the compound that has an M 1 ion at mÜz 136 in its CI mass spectrum, an IR 1 + = absorption at 3279 cm_ , and the following NMR spectrum: d 0.91 (1H, s), d 1.07 (3H, t, J 7Hz), d 2.60 (2H, q, J 7Hz), d 3.70 (2H, s), d 7.18 (5H, apparent s). -

Physicochemical Properties of Organic Medicinal Agents
Principles of Drug Action 1, Spring 2005, Esters ESTERS AND RELATED CARBOXYLIC ACID DERIVATIVES Jack DeRuiter I. Structure and Preparation Esters are derivatives of carboxylic acids that arise via replacement of the hydroxyl (OH) portion of the acid COOH function with an "ether" moiety (-OR): O O H C C O C O Acid Ester Note that replacement of the acid OH group with an "ether" moiety removes the acidic function from the parent structure (acid) resulting in the formation of non-acidic (neutral, but somewhat polar) compounds (esters). Esters can be sub-classified based on their general structure as aliphatic, aromatic or cyclic (called "lactones") as illustrated by the examples below: O O CH2CH3 CH2CH3 O CH3 O O O Aliphatic Ester Aromatic Ester Cyclic Ester (Lactone) A variety of methods have been developed for the preparation of esters. Most of these methods involve reaction of an alcohol with an "activated carboxylic acid" compound (i.e. acid chloride): O O H C C X OC C O X- Ester "Activated" acid (X=Cl) Alcohol (Electrophile) (Nucleophile) The ester functionality does not introduce a center of asymmetry and thus optical and geometric isomerism does not result from the presence of this functional group. The ester functionality (the carbonyl and ether oxygen) is composed of an sp2 hybridized carbon so it cannot be chiral, and since there is free rotation about the ether bond geometric isomerism also is not possible at the sp2 center. 1 Principles of Drug Action 1, Spring 2005, Esters II. Solubility of Esters Esters contain carbonyl (C=O) and ether (O-C) dipoles arising from covalent bonding between electronegative oxygen atoms and electronically neutral carbon atoms. -

Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely Through a Common Mechanism S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/03/21/mol.117.108290.DC1 1521-0111/91/6/620–629$25.00 https://doi.org/10.1124/mol.117.108290 MOLECULAR PHARMACOLOGY Mol Pharmacol 91:620–629, June 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely through a Common Mechanism s Anita Luethy, James D. Boghosian, Rithu Srikantha, and Joseph F. Cotten Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts (A.L., J.D.B., and J.F.C.); Department of Anesthesia, Kantonsspital Aarau, Aarau, Switzerland (A.L.); Carver College of Medicine, University of Iowa, Iowa City, Iowa (R.S.) Received January 7, 2017; accepted March 20, 2017 Downloaded from ABSTRACT The TWIK-related acid-sensitive potassium channel 3 (TASK-3; hydrate (165% [161–176]) . 2,2-dichloro- . 2-chloro 2,2,2- KCNK9) tandem pore potassium channel function is activated by trifluoroethanol . ethanol. Similarly, carbon tetrabromide (296% halogenated anesthetics through binding at a putative anesthetic- [245–346]), carbon tetrachloride (180% [163–196]), and 1,1,1,3,3,3- binding cavity. To understand the pharmacologic requirements for hexafluoropropanol (200% [194–206]) activate TASK-3, whereas molpharm.aspetjournals.org TASK-3 activation, we studied the concentration–response of the larger carbon tetraiodide and a-chloralose inhibit. Clinical TASK-3 to several anesthetics (isoflurane, desflurane, sevoflurane, agents activate TASK-3 with the following rank order efficacy: halothane, a-chloralose, 2,2,2-trichloroethanol [TCE], and chloral halothane (207% [202–212]) . -

BENZENE AS a LARVICIDE for SCREW WORMS1 the Larval Stage
BENZENE AS A LARVICIDE FOR SCREW WORMS1 By D. C. PARMAN Assistant Entomologist, Investigations of Insects Affecting the Health of Animals, Bureau of Entomology, United States Department of Agriculture INTRODUCTION The larval stage of CocMiomyia macellaria Fab., generally known amonff stock raisers in the Southwest as the screw worm, causes con- siderable loss to the livestock industry, estimated as high as $5,000,000 in some years. It has been apparent that the larvicides used to kill the worms are either toxic to the animal or at least in most cases detrimental to the healing of the wounds. This toxicity was at first attributed to the screw worm, but as many cases were ob- served where the animal was practically consumed by the larvae and still lived until the loss of olood or injury to some vital organ brought de^th, it was surmised that the treatments with larvicides were the cause of many deaths. During the summer of 1916 syste- matic work was begun to find a more efficient larvicide than the phenols and chloroform which were generally used. At first an attempt was made to add something to these larvicides to counteract the toxic properties. As this was not successful it was deemed best to look for a chemical that might be used with more satis- factory results. Several chemical groups were studied for possible larvicides. EXPERIMENTAL PROCEDURE All available chemicals with possible larvicidal value were selected for laboratory tests to determine whether they would kill the larvae of the screw-worm fly. The first tests were made by pouring the chemical on a number of larvae in a tube, or dusting on just enough to cover the larvae. -

2 Reactions Observed with Alkanes Do Not Occur with Aromatic Compounds 2 (SN2 Reactions Never Occur on Sp Hybridized Carbons!)
Reactions of Aromatic Compounds Aromatic compounds are stabilized by this “aromatic stabilization” energy Due to this stabilization, normal SN2 reactions observed with alkanes do not occur with aromatic compounds 2 (SN2 reactions never occur on sp hybridized carbons!) In addition, the double bonds of the aromatic group do not behave similar to alkene reactions Aromatic Substitution While aromatic compounds do not react through addition reactions seen earlier Br Br Br2 Br2 FeBr3 Br With an appropriate catalyst, benzene will react with bromine The product is a substitution, not an addition (the bromine has substituted for a hydrogen) The product is still aromatic Electrophilic Aromatic Substitution Aromatic compounds react through a unique substitution type reaction Initially an electrophile reacts with the aromatic compound to generate an arenium ion (also called sigma complex) The arenium ion has lost aromatic stabilization (one of the carbons of the ring no longer has a conjugated p orbital) Electrophilic Aromatic Substitution In a second step, the arenium ion loses a proton to regenerate the aromatic stabilization The product is thus a substitution (the electrophile has substituted for a hydrogen) and is called an Electrophilic Aromatic Substitution Energy Profile Transition states Transition states Intermediate Potential E energy H Starting material Products E Reaction Coordinate The rate-limiting step is therefore the formation of the arenium ion The properties of this arenium ion therefore control electrophilic aromatic substitutions (just like any reaction consider the stability of the intermediate formed in the rate limiting step) 1) The rate will be faster for anything that stabilizes the arenium ion 2) The regiochemistry will be controlled by the stability of the arenium ion The properties of the arenium ion will predict the outcome of electrophilic aromatic substitution chemistry Bromination To brominate an aromatic ring need to generate an electrophilic source of bromine In practice typically add a Lewis acid (e.g. -

Hydroxylamine-O -Sulfonic Acid — a Versatile Synthetic Reagent
Hydroxylamine-O -sulfonic acid — a versatile synthetic reagent Raymond G. Wallacef School of Chemistry Brunei University Uxbridge, Middlesex UBS 3PH Great Britain imidazoli nones and related derivatives are time to these various modes of reaction. discussed in the review. Many of these The uses of HOSA as a reagent are organiz preparations can be carried out in high ed below according to the different syn yield, thetic transformations that it can bring about. Hydroxylamine-Osulfonic acid, NHj-OSOjH (abbreviated to HOSA in Probably by far the most well known this article) has become in recent years and explored reactions of HOSA are commercially available. Although much animation reactions, illustrating elec fruitful chemistry has been carried out us trophilic attack by HOSA, with amination ing HOSA, to this author's knowledge, on nitrogen being the most important, there has been no systematic review in although a significant number of English* of its use as a synthetic reagent. It animations on both carbon and sulfur have is a chemically interesting compound been reported, Amination on phosphorus because of the ability of the nitrogen center also occurs. to act in the role of both nucleophile and AMINATION electrophile, dependent on circumstances, Synopsis (a) At a nitrogen atom and thus it has proved to be a reagent of Hydroxylamine-0-sulfonic acid (0 Preparation of mono- and di- great synthetic versatility. (HOSA) has only recently become widely substituted hydrazines and trisubstituied commercially available despite the fact that H,N-Nu hydrazinium salts it has proved to be a valuable synthetic reagent in preparative organic chemistry. -

Chemistry and Physics of Lipids Effects of Ether Vs. Ester Linkage On
Chemistry and Physics of Lipids 160 (2009) 33–44 Contents lists available at ScienceDirect Chemistry and Physics of Lipids journal homepage: www.elsevier.com/locate/chemphyslip Effects of ether vs. ester linkage on lipid bilayer structure and water permeability S. Deren Guler a, D. Dipon Ghosh b, Jianjun Pan a, John C. Mathai c, Mark L. Zeidel c, John F. Nagle a,b, Stephanie Tristram-Nagle a,∗ a Department of Physics, Carnegie Mellon University, Pittsburgh, PA 15213, USA b Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, PA 15213, USA c Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Cambridge, MA 02139, USA article info abstract Article history: The structure and water permeability of bilayers composed of the ether-linked lipid, dihexadecylphos- Received 29 January 2009 phatidylcholine (DHPC), were studied and compared with the ester-linked lipid, dipalmitoylphosphadit- Received in revised form 29 March 2009 dylcholine (DPPC). Wide angle X-ray scattering on oriented bilayers in the fluid phase indicate that the Accepted 26 April 2009 area per lipid A is slightly larger for DHPC than for DPPC. Low angle X-ray scattering yields A = 65.1 Å2 for Available online 3 May 2009 ◦ −13 DHPC at 48 C. LAXS data provide the bending modulus, KC = 4.2 × 10 erg, and the Hamaker parame- ter H =7.2× 10−14 erg for the van der Waals attractive interaction between neighboring bilayers. For the Keywords: low temperature phases with ordered hydrocarbon chains, we confirm the transition from a tilted L Ether lipid ◦ gel phase to an untilted, interdigitated LI phase as the sample hydrates at 20 C. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -
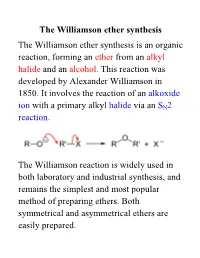
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube. -

Educational Research Applications Abebe M, Et Al
Educational Research Applications Abebe M, et al. Educ Res Appl 5: 175. Review Article DOI: 10.29011/2575-7032.100175 Teaching Students Synthesizing Molecules Mimicking an Existing Drug against Covid-19 Moges Abebe1*, Lashan Eloise Knowles1, Bisrat Hailemeskel2 1Department of Biological and Physical Sciences, Saint Augustine University, Raleigh, NC, USA 2Department of Clinical & Administrative Pharmacy Sciences, College of Pharmacy, Howard University, NW Washington, DC, USA *Corresponding author: Moges Abebe, Department of Biological and Physical Sciences, Saint Augustine University, Raleigh, NC 27610, NC, USA Citation: Abebe M, Knowles LE, Hailemeskel B (2020) Teaching Students Synthesizing Molecules Mimicking an Existing Drug against Covid-19. Educ Res Appl 5: 175. DOI: 10.29011/2575-7032.100175 Received Date: 26 May 2020; Accepted Date: 01 June, 2020; Published Date: 06 June, 2020 Abstract End of semester organic chemistry course projects are valuable learning assessment tools while giving students a creative opportunity and sparking interest for further research investigations. The purpose of this year’s project was to teach students how to synthesize a molecule that potentially mimics an existing drug that works against the COVID-19. The available drugs chosen for the project are those that are proposed to work either by prohibiting the easy entry of the virus into respiratory tissues or those who deprive the virus’s ability to reproduce once they enter the cell. An investigative search in historical literature and the current conditions of the virus enabled students to create a unique and innovative product that requires a cumulative learned knowledge. History has shown that when a new virus becomes pandemic it takes time for researchers to create a drug, test the results, and gets approved by the Food and Drug Administration (FDA) for public availability.