The Large Temporal Horn: MR Analysis in Developmental Brain Anomalies Versus Hydrocephalus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fetal Brain Anomalies Associated with Ventriculomegaly Or Asymmetry: an MRI-Based Study
ORIGINAL RESEARCH PEDIATRICS Fetal Brain Anomalies Associated with Ventriculomegaly or Asymmetry: An MRI-Based Study X E. Barzilay, X O. Bar-Yosef, X S. Dorembus, X R. Achiron, and X E. Katorza ABSTRACT BACKGROUND AND PURPOSE: Fetal lateral ventriculomegaly is a relatively common finding with much debate over its clinical signifi- cance. The purpose of this study was to examine the association between ventriculomegaly and asymmetry and concomitant CNS findings as seen in fetal brain MR imaging. MATERIALS AND METHODS: Fetal brain MR imaging performed for various indications, including ventriculomegaly, with or without additional ultrasound findings, was assessed for possible inclusion. Two hundred seventy-eight cases found to have at least 1 lateral ventricle with a width of Ն10 mm were included in the study. Ventriculomegaly was considered mild if the measurement was 10–11.9 mm; moderate if, 12–14.9 mm; and severe if, Ն15 mm. Asymmetry was defined as a difference of Ն2 mm between the 2 lateral ventricles. Fetal brain MR imaging findings were classified according to severity by predefined categories. RESULTS: The risk of CNS findings appears to be strongly related to the width of the ventricle (OR, 1.38; 95% CI, 1.08–1.76; P ϭ .009). The prevalence of associated CNS abnormalities was significantly higher (P ϭ .005) in symmetric ventriculomegaly compared with asymmetric ventriculomegaly (38.8% versus 24.2%, respectively, for all CNS abnormalities and 20% versus 7.1%, respectively, for major CNS abnormalities). CONCLUSIONS: In this study, we demonstrate that the rate of minor and major findings increased with each millimeter increase in ventricle width and that the presence of symmetric ventricles in mild and moderate ventriculomegaly was a prognostic indicator for CNS abnormalities. -

Telovelar Approach to the Fourth Ventricle: Microsurgical Anatomy
J Neurosurg 92:812–823, 2000 Telovelar approach to the fourth ventricle: microsurgical anatomy ANTONIO C. M. MUSSI, M.D., AND ALBERT L. RHOTON, JR., M.D. Department of Neurological Surgery, University of Florida, Gainesville, Florida Object. In the past, access to the fourth ventricle was obtained by splitting the vermis or removing part of the cere- bellum. The purpose of this study was to examine the access to the fourth ventricle achieved by opening the tela cho- roidea and inferior medullary velum, the two thin sheets of tissue that form the lower half of the roof of the fourth ven- tricle, without incising or removing part of the cerebellum. Methods. Fifty formalin-fixed specimens, in which the arteries were perfused with red silicone and the veins with blue silicone, provided the material for this study. The dissections were performed in a stepwise manner to simulate the exposure that can be obtained by retracting the cerebellar tonsils and opening the tela choroidea and inferior medullary velum. Conclusions. Gently displacing the tonsils laterally exposes both the tela choroidea and the inferior medullary velum. Opening the tela provides access to the floor and body of the ventricle from the aqueduct to the obex. The additional opening of the velum provides access to the superior half of the roof of the ventricle, the fastigium, and the superolater- al recess. Elevating the tonsillar surface away from the posterolateral medulla exposes the tela, which covers the later- al recess, and opening this tela exposes the structure forming -

Pediatric Hydrocephalus; a Statistical and Historical Approach by R
Global Journal of Medical Research: A Neurology and Nervous System Volume 15 Issue 1 Version 1.0 Year 2015 Type: Double Blind Peer Reviewed International Research Journal Publisher: Global Journals Inc. (USA) Online ISSN: 2249-4618 & Print ISSN: 0975-5888 Pediatric Hydrocephalus; A Statistical and Historical Approach By R. Omidi-Varmezani University of Spital Buelach- Zurich, Switzerland Abstract- Purpose: The purpose of this study was to determine the clinical statistical collection of pediatric hydrocephalus with emphasis on pathology to investigate the epidemiology of pediatric hydrocephalus. Materials and Methods: We performed respectively analysis of the pediatric patients younger than 18 years with or suspected of hydrocephalus in our neuropediatric centre since 2001. Data were obtained from our neuropediatric centre database and from hospital admission records of the university hospital of Homburg. After the patient selection procedure and sort out the hydrocephalus patients were a total of 193 children registered at the University Hospital of Homburg Children Medical Centre, between June 2001 and March 2009. The patients were under age and mechanism of hydrocephalus distributed. Keywords: hydrocephalus, peditric neuroradiology, intracerebral pressure. GJMR-A Classification : NLMC Code: WM 170 PediatricHydrocephalusAStatisticalandHistoricalApproach Strictly as per the compliance and regulations of: © 2015. R. Omidi-Varmezani. This is a research/review paper, distributed under the terms of the Creative Commons Attribution- Noncommercial 3.0 Unported License http://creativecommons.org/licenses/by-nc/3.0/), permitting all non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Pediatric Hydrocephalus; A Statistical and Historical Approach R. Omidi-Varmezani Abstract- Purpose: The purpose of this study was to determine can flow from the 4th ventricle into the subarachnoidal the clinical statistical collection of pediatric hydrocephalus with space through two apertures. -

Locus Coeruleus Complex of the Family Delphinidae
www.nature.com/scientificreports OPEN Locus coeruleus complex of the family Delphinidae Simona Sacchini 1, Manuel Arbelo 1, Cristiano Bombardi2, Antonio Fernández1, Bruno Cozzi 3, Yara Bernaldo de Quirós1 & Pedro Herráez1 Received: 19 July 2017 The locus coeruleus (LC) is the largest catecholaminergic nucleus and extensively projects to widespread Accepted: 22 March 2018 areas of the brain and spinal cord. The LC is the largest source of noradrenaline in the brain. To date, the Published: xx xx xxxx only examined Delphinidae species for the LC has been a bottlenose dolphin (Tursiops truncatus). In our experimental series including diferent Delphinidae species, the LC was composed of fve subdivisions: A6d, A6v, A7, A5, and A4. The examined animals had the A4 subdivision, which had not been previously described in the only Delphinidae in which this nucleus was investigated. Moreover, the neurons had a large amount of neuromelanin in the interior of their perikarya, making this nucleus highly similar to that of humans and non-human primates. This report also presents the frst description of neuromelanin in the cetaceans’ LC complex, as well as in the cetaceans’ brain. Te locus coeruleus (LC) is a densely packed cluster of noradrenaline-producing neurons located in the upper part of the rostral rhombencephalon, on the lateral edge of the fourth ventricle. Te LC is the largest catechola- minergic nucleus of the brain, and it supplies noradrenaline to the entire central nervous system. Noradrenaline neurons are located in the medulla oblongata and pons (termed A1-A7 divisions), while adrenaline neurons are located only in the medulla oblongata, near A1-A3 (and termed C1-C3)1. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Surgical Anatomy and Techniques
SURGICAL ANATOMY AND TECHNIQUES MICROSURGICAL APPROACHES TO THE MEDIAL TEMPORAL REGION:AN ANATOMICAL STUDY Alvaro Campero, M.D. OBJECTIVE: To describe the surgical anatomy of the anterior, middle, and posterior Department of Neurological Surgery, portions of the medial temporal region and to present an anatomic-based classification University of Florida, of the approaches to this area. Gainesville, Florida METHODS: Twenty formalin-fixed, adult cadaveric specimens were studied. Ten brains Gustavo Tro´ccoli, M.D. provided measurements to compare different surgical strategies. Approaches were demon- Department of Neurological Surgery, strated using 10 silicon-injected cadaveric heads. Surgical cases were used to illustrate the Hospital “Dr. J. Penna,” results by the different approaches. Transverse lines at the level of the inferior choroidal point Bahı´a Blanca, Argentina and quadrigeminal plate were used to divide the medial temporal region into anterior, middle, and posterior portions. Surgical approaches to the medial temporal region were classified into Carolina Martins, M.D. four groups: superior, lateral, basal, and medial, based on the surface of the lobe through which Department of Neurological Surgery, University of Florida, the approach was directed. The approaches through the medial group were subdivided further Gainesville, Florida into an anterior approach, the transsylvian transcisternal approach, and two posterior ap- proaches, the occipital interhemispheric and supracerebellar transtentorial approaches. Juan C. Fernandez-Miranda, M.D. RESULTS: The anterior portion of the medial temporal region can be reached through Department of Neurological Surgery, University of Florida, the superior, lateral, and basal surfaces of the lobe and the anterior variant of the Gainesville, Florida approach through the medial surface. -

Morphometric Assesment of the External Anatomy of Fourth Ventricle and Dorsal Brainstem in Fresh Cadavers
DOI: 10.5137/1019-5149.JTN.24942-18.1 Turk Neurosurg 29(3):445-450, 2019 Received: 26.09.2018 / Accepted: 20.11.2018 Published Online: 19.12.2018 Original Investigation Morphometric Assesment of the External Anatomy of Fourth Ventricle and Dorsal Brainstem in Fresh Cadavers Veysel ANTAR1, Okan TURK1, Salim KATAR2, Mahmut OZDEN3, Balkan SAHIN4, Sahin YUCELI5, Erdogan KARA6, Ayse YURTSEVEN6 1Istanbul Training and Research Hospital, Department of Neurosurgery, Istanbul, Turkey 2Selahattin Eyyubi City Hospital, Department of Neurosurgery, Diyarbakir, Turkey 3Bahcesehir University, Department of Neurosurgery, Istanbul, Turkey 4Sultan Abdulhamit Han Training and Research Hospital, Department of Neurosurgery, Istanbul, Turkey 5Erzincan Neon Hospital, Department of Neurosurgery, Erzincan, Turkey 6Ministry of Justice, Council of Forensic Medicine, Istanbul, Turkey Corresponding author: Veysel ANTAR [email protected] ABSTRACT AIM: To investigate the external anatomy of the fourth ventricle and dorsal brainstem using morphometric data, which could be useful for preoperative surgical planning. MATERIAL and METHODS: Between January 2017 and December 2017, 42 fresh adult cadavers were investigated for the measurements of the cadaver brainstems and fourth ventricle, and they were recorded by photography. Measurements were evaluated according to body mass indexes (BMIs) of the patients. We also investigate the visualization of facial colliculus and stria medullaris on brainstem. RESULTS: A total of 42 fresh cadavers with a mean age of 45.38 ± 16.41 years old were included in this research. We found no statistically significant difference between measurements and BMIs. Facial colliculus was visualized in 92.9% (n=39), but it could not visualized in 7.1% (n=3) of the subjects. -

Ventriculomegaly
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Ventriculomegaly This information sheet from Great Ormond Street Hospital (GOSH) explains the causes, symptoms and treatment of ventriculomegaly and hydrocephalus and where to get help. Ventricles are cavities within the brain filled Without signs of increased pressure in the with cerebro-spinal fluid (CSF) acting as a brain (hydrocephalus), ventriculomegaly most ‘cushion’. CSF also supplies nutrients to the likely will not cause any problems. However, brain. The brain has four ventricles: two it can be linked with hydrocephalus and other lateral ventricles, the third ventricle and the problems. Ventriculomegaly can be diagnosed fourth ventricle. during pregnancy and occurs in around two CSF is created within the brain and flows from per cent of all pregnancies. the lateral ventricles into the third ventricle. It then flows through a narrow tube (the What causes cerebral aqueduct) into the fourth ventricle which lies towards the base of the brain. From ventriculomegaly? the fourth ventricle, it flows around the spinal In many cases, we do not know what causes cord and over the surface of the brain before ventriculomegaly (in the absence of any raised being re-absorbed. CSF pressure) but it can occur if there has been Ventriculomegaly is the medical term used to brain damage for any reason leading to loss describe enlargement of the ventricles of the of brain tissue. Often however it is a “chance” brain. Hydrocephalus is the term used when finding and when the ventricles are only a enlargement of the ventricles has been caused little enlarged of little significance. -

Morphometric Analysis of Ventricular System of Human Brain - a Study by Dissection Method
Jemds.com Original Research Article Morphometric Analysis of Ventricular System of Human Brain - A Study by Dissection Method Prabahita Baruah1, Purujit Choudhury2, Pradipta Ray Choudhury3 1Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. 2Department of Surgery, Gauhati Medical College and Hospital, Guwahati, Assam, India. 3Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. ABSTRACT BACKGROUND It is often a challenge to determine if the brain ventricles are within normal limits Corresponding Author: or swollen with the age of the patient. With a standardized and comparable system, Dr. Purujit Choudhury, it is therefore necessary to define normal ventricular size ranges. Cadaveric Associate Professor, dissection is always considered the gold standard of anatomical education. Present Department of Surgery, Gauhati Medical College and Hospital, work is undertaken to study morphometric analysis of lateral, third & fourth Guwahati, Assam, India. ventricles by dissection method. Morphometric assessment of the ventricular E-mail: [email protected] system is helpful in the diagnosis as well as classification of hydrocephalus and in the evaluation and monitoring of ventricular system enlargement during DOI: 10.14260/jemds/2020/121 ventricular shunt therapy. Financial or Other Competing Interests: METHODS None. Different parameters of all parts of lateral ventricle, third and fourth ventricle were How to Cite This Article: measured with digital vernier caliper in cadaveric brain specimens. The brain Baruah P, Choudhury P, Choudhury PR. specimens were obtained from dead bodies subjected to post-mortem Morphometric analysis of ventricular examinations in the Department of Forensic Medicine and from the dead bodies system of human brain- a study by voluntarily donated to the Department of Anatomy, Silchar Medical College, Silchar. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -

Is Composed from Spinal Cord and Brain
doc. MUDr. Adriana Boleková, PhD. MVDr. Natália Hvizdošová, PhD. CENTRAL NERVOUS SYSTEM – is composed from spinal cord and brain SPINAL CORD cranial border: foramen magnum, pyramidal decussation, exit of first pair of spinal nerves caudal border: level of L1 – L2 vertebrae medullary cone – filum terminale (S2) – cauda equina enlargements: cervical enlargement (C5 – Th1): origin of nerves for upper extremity – brachial plexus lumbosacral enlargement (L1 – S2): origin of nerves for lower extremity – lumbosacral plexus external features: anterior median fissure anterolateral sulcus – anterior roots of spinal nn. posterolateral sulcus – posterior roots of spinal nn. posterior median sulcus posterior intermediate sulcus internal features: White matter anterior funiculus (between anterior median fissure and anterolateral sulcus) lateral funiculus (between anterolateral and posterolateral sulci) posterior funiculus (between posterolateral sulcus and posterior median sulcus) fasciculus gracilis fasciculus cuneatus Gray matter anterior (ventral) horn – motor function: Rexed laminae I – VI lateral horn – serves to visceral function: Rexed lamina VII dorsal (posterior) horn – sensory information: Rexed laminae VIII – IX central grey matter – interneurons: around central canal Rexed lamina X Central canal cranially opens into IV. ventricle caudally expands into terminal ventricle vessels of spinal cord: Arteries: spinal brr. from surrounding arteries – anterior radicular aa., posterior radicular aa.: posterior spinal aa. (in posterolateral -
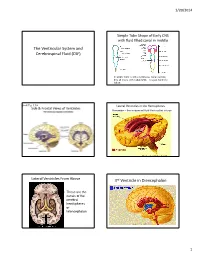
The Ventricular System and Cerebrospinal Fluid (CSF) 3Rd Ventricle in Diencephalon
1/20/2014 Simple Tube Shape of Early CNS with fluid filled canal in middle The Ventricular System and Cerebrospinal Fluid (CSF) In adults there is still a continuous canal running thru all levels of the adult CNS – it is just harder to follow. Book Fig. 1.19 Lateral Ventricles in the Hemispheres Side & Frontal Views of Ventricles Remember – these represent fluid filled cavities in brain “Wishbone” shape means there is ventricle within each of the lobes. Lateral Ventricles From Above 3rd Ventricle in Diencephalon • These are the canals of the cerebral hemispheres or telencephalon 1 1/20/2014 Interventricular foramen links lateral ventricles to 3rd You can see the 2 dark lateral ventricles as well as the vertical midline 3 rd ventricle Sagittal Section Thru 3 rd Vent. (#2 in figure) • Connects to skinny cerebral aqueduct, the canal of the midbrain (just under the #10) Choroid plexus located in ventricles continuously 4th Ventricle in the Hindbrain produces CSF, replacing the ~125-150 ml several times/day Normal pressure 3 holes in the roof of CSF: of the 4 th venticle 100-150 mmH2O allow most CSF to lying down leave ventricles 200-300 mmH2O and enter the sitting up subarachnoid space around brain 2 1/20/2014 Circulation of CSF in • Why is CSF pressure measured in mm H2O? • Larger pressure differences (like BP) are ventricles measured by the movement of a column of always heavier liquid (Mercury or Hg) moves • Smaller pressure differences are measured by the movement of a column of lighter liquid (H2O) posteriorly, – CSF moves that column ~150 mm but would toward only move Hg about 2 mm.