Growing Evening Primroses (Oenothera)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vestured Pits in Wood of Onagraceae: Correlations with Ecology, Habit, and Phylogeny1
VESTURED PITS IN WOOD OF Sherwin Carlquist2 and Peter H. Raven3 ONAGRACEAE: CORRELATIONS WITH ECOLOGY, HABIT, AND PHYLOGENY1 ABSTRACT All Onagraceae for which data are available have vestured pits on vessel-to-vessel pit pairs. Vestures may also be present in some species on the vessel side of vessel-to-ray pit pairs. Herbaceous Onagraceae do not have fewer vestures, although woods with lower density (Circaea L. and Oenothera L.) have fewer vestures. Some Onagraceae from drier areas tend to have smaller vessel pits, and on that account may have fewer vestures (Epilobium L. and Megacorax S. Gonz´alez & W. L. Wagner). Pit apertures as seen on the lumen side of vessel walls are elliptical, occasionally oval, throughout the family. Vestures are predominantly attached to pit aperture margins. As seen from the outer surfaces of vessels, vestures may extend across the pit cavities. Vestures are usually absent or smaller on the distal portions of pit borders (except for Ludwigia L., which grows consistently in wet areas). Distinctive vesture patterns were observed in the several species of Lopezia Cav. and in Xylonagra Donn. Sm. & Rose. Vestures spread onto the lumen-facing vessel walls of Ludwigia octovalvis (Jacq.) P. H. Raven. Although the genera are presented here in the sequence of a recent molecular phylogeny of Onagraceae, ecology and growth forms are more important than evolutionary relationships with respect to abundance, degree of grouping, and morphology of vestured pits. Designation of vesture types is not warranted based on the distribution of named types in Onagraceae and descriptive adjectives seem more useful, although more data on vesturing in the family are needed before patterns of diversity and their extent can be fully ascertained. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

GARDENERGARDENER® Thethe Magazinemagazine Ofof Thethe Aamericanmerican Horticulturalhorticultural Societysociety July / August 2007
TheThe AmericanAmerican GARDENERGARDENER® TheThe MagazineMagazine ofof thethe AAmericanmerican HorticulturalHorticultural SocietySociety July / August 2007 pleasures of the Evening Garden HardyHardy PlantsPlants forfor Cold-ClimateCold-Climate RegionsRegions EveningEvening PrimrosesPrimroses DesigningDesigning withwith See-ThroughSee-Through PlantsPlants WIN THE BATTLE OF THE BULB The OXO GOOD GRIPS Quick-Release Bulb Planter features a heavy gauge steel shaft with a soft, comfortable, non-slip handle, large enough to accommodate two hands. The Planter’s patented Quick-Release lever replaces soil with a quick and easy squeeze. Dig in! 1.800.545.4411 www.oxo.com contents Volume 86, Number 4 . July / August 2007 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 7 NEWS FROM AHS AHS award winners honored, President’s Council trip to Charlotte, fall plant and antiques sale at River Farm, America in Bloom Symposium in Arkansas, Eagle Scout project enhances River Farm garden, second AHS page 7 online plant seminar on annuals a success, page 39 Homestead in the Garden Weekend. 14 AHS PARTNERS IN PROFILE YourOutDoors, Inc. 16 PLEASURES OF THE EVENING GARDEN BY PETER LOEWER 44 ONE ON ONE WITH… Enjoy the garden after dark with appropriate design, good lighting, and the addition of fragrant, night-blooming plants. Steve Martino, landscape architect. 46 NATURAL CONNECTIONS 22 THE LEGEND OF HIDDEN Parasitic dodder. HOLLOW BY BOB HILL GARDENER’S NOTEBOOK Working beneath the radar, 48 Harald Neubauer is one of the Groundcovers that control weeds, meadow rues suited for northern gardens, new propagation wizards who online seed and fruit identification guide, keeps wholesale and retail national “Call Before You Dig” number nurseries stocked with the lat- established, saving wild magnolias, Union est woody plant selections. -

Biological and Host Range Characteristics of Lysathia Flavipes
insects Article Biological and Host Range Characteristics of Lysathia flavipes (Coleoptera: Chrysomelidae), a Candidate Biological Control Agent of Invasive Ludwigia spp. (Onagraceae) in the USA Angelica M. Reddy 1,* , Paul D. Pratt 1, Brenda J. Grewell 2 , Nathan E. Harms 3, Ximena Cibils-Stewart 4, Guillermo Cabrera Walsh 5 and Ana Faltlhauser 5,6 1 USDA-ARS, Invasive Species and Pollinator Health Research Unit, Western Regional Research Center, 800 Buchanan St., Albany, CA 94710, USA; [email protected] 2 USDA-ARS, Invasive Species and Pollinator Health Research Unit, University of California Davis, Department of Plant Sciences Mail Stop 4, One Shields Ave, Davis, CA 95616, USA; [email protected] 3 US Army Engineer Research and Development Center (ERDC), Aquatic Ecology and Invasive Species Branch, 3909 Halls Ferry Rd, Vicksburg, MS 39180, USA; [email protected] 4 Instituto Nacional de Investigación Agropecuaria (INIA), Estación Experimental INIA La Estanzuela, Ruta 50 Km 11, Colonia del Sacramento, Colonia, Uruguay; [email protected] 5 Fundación para el Estudio de Especies Invasivas (FuEDEI), Simón Bolívar 1559, Hurlingham (CP1686), Buenos Aires B1686EFA, Argentina; [email protected] (G.C.W.); [email protected] (A.F.) 6 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Godoy Cruz 2290, Ciudad Autónoma de Buenos Aires C1425FQB, Argentina * Correspondence: [email protected] Citation: Reddy, A.M.; Pratt, P.D.; Grewell, B.J.; Harms, N.E.; Simple Summary: Exotic water primroses (Ludwigia spp.) are aggressive plant invaders in aquatic Cibils-Stewart, X.; Cabrera Walsh, G.; Faltlhauser, A. Biological and Host ecosystems worldwide. Management of exotic Ludwigia spp. -

Levin Et Al. 2004
Systematic Botany (2004), 29(1): pp. 147–164 q Copyright 2004 by the American Society of Plant Taxonomists Paraphyly in Tribe Onagreae: Insights into Phylogenetic Relationships of Onagraceae Based on Nuclear and Chloroplast Sequence Data RACHEL A. LEVIN,1,7 WARREN L. WAGNER,1 PETER C. HOCH,2 WILLIAM J. HAHN,3 AARON RODRIGUEZ,4 DAVID A. BAUM,5 LILIANA KATINAS,6 ELIZABETH A. ZIMMER,1 and KENNETH J. SYTSMA5 1Department of Systematic Biology, Botany, MRC 166, Smithsonian Institution, P. O. Box 37012, Washington, District of Columbia 20013-7012; 2Missouri Botanical Garden, P. O. Box 299, St. Louis, Missouri 63166-0299; 3108 White-Gravenor, Box 571003, Georgetown University, Washington, District of Columbia, 20057-1003; 4Departamento de Botan´‡ca y Zoolog´‡a, Apartado Postal 139, 45101 Zapopan, Jalisco, Mexico; 5Department of Botany, University of Wisconsin, 430 Lincoln Drive, Madison, Wisconsin 53706; 6Departamento Cienti!co de Plantas Vasculares, Museo de Ciencias Naturales, Paseo del Bosque s/n, 1900 La Plata, Provincia de Buenos Aires, Argentina 7Author for correspondence ([email protected]) Communicating Editor: Thomas G. Lammers ABSTRACT. Onagraceae are a family of 17 genera in seven tribes, with the majority of species in tribes Onagreae and Epilobieae. Despite the species-richness of these two tribes, to date no phylogenetic study has been done with suf!cient taxon sampling to examine relationships between and within these tribes. In this study, we used DNA sequence data from one nuclear region (ITS) and two chloroplast regions (trnL-trnF and rps16) to infer phylogenetic relationships among 93 taxa across the family, with concentrated sampling in the large tribe Onagreae. -

Vascular Plant Species of the Comanche National Grassland in United States Department Southeastern Colorado of Agriculture
Vascular Plant Species of the Comanche National Grassland in United States Department Southeastern Colorado of Agriculture Forest Service Donald L. Hazlett Rocky Mountain Research Station General Technical Report RMRS-GTR-130 June 2004 Hazlett, Donald L. 2004. Vascular plant species of the Comanche National Grassland in southeast- ern Colorado. Gen. Tech. Rep. RMRS-GTR-130. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 36 p. Abstract This checklist has 785 species and 801 taxa (for taxa, the varieties and subspecies are included in the count) in 90 plant families. The most common plant families are the grasses (Poaceae) and the sunflower family (Asteraceae). Of this total, 513 taxa are definitely known to occur on the Comanche National Grassland. The remaining 288 taxa occur in nearby areas of southeastern Colorado and may be discovered on the Comanche National Grassland. The Author Dr. Donald L. Hazlett has worked as an ecologist, botanist, ethnobotanist, and teacher in Latin America and in Colorado. He has specialized in the flora of the eastern plains since 1985. His many years in Latin America prompted him to include Spanish common names in this report, names that are seldom reported in floristic pub- lications. He is also compiling plant folklore stories for Great Plains plants. Since Don is a native of Otero county, this project was of special interest. All Photos by the Author Cover: Purgatoire Canyon, Comanche National Grassland You may order additional copies of this publication by sending your mailing information in label form through one of the following media. -

Transferability of Microsatellite Markers Developed in Oenothera Spp
diversity Communication Transferability of Microsatellite Markers Developed in Oenothera spp. to the Invasive Species Oenothera drummondii Hook. (Onagraceae) Raquel Hernández-Espinosa 1, Jorge González-Astorga 1,* , Alejandro Espinosa de los Monteros 1 ,Dánae Cabrera-Toledo 2 and Juan B. Gallego-Fernández 3 1 Red de Biología Evolutiva, Instituto de Ecología A. C. Carretera Antigua a Coatepec No. 351, El Haya, Xalapa 91073, Veracruz, Mexico; [email protected] (R.H.-E.); [email protected] (A.E.d.l.M.) 2 Instituto de Botánica, Departamento de Botánica y Zoología, Centro Universitario de Ciencias Biológicas y Agropecuarias, Universidad de Guadalajara, Zapopan 44600, Mexico; [email protected] 3 Departamento de Biología Vegetal y Ecología, Universidad de Sevilla, 41012 Sevilla, Spain; [email protected] * Correspondence: [email protected]; Tel.: +52-1-228-307-8097 Received: 31 August 2020; Accepted: 6 October 2020; Published: 8 October 2020 Abstract: Oenothera drummondii Hook. (Onagraceae) has life-history traits that make it an invasive species. Native populations are distributed along the coastal dunes from North Carolina in the United States to Tabasco in the Gulf of Mexico. It has been reported as an invasive species in Spain, Israel, and China, where this species can successfully colonize and dominate if the environmental conditions are appropriate. In South Africa, Australia, New Zealand, and France, it is reported to be naturalized. In this study, 28 microsatellite markers developed for other Oenothera species were evaluated for cross-amplification in O. drummondii. Nine primers showed consistent amplification and were polymorphic. Polymorphism was assessed in three populations from both native and invaded areas. -
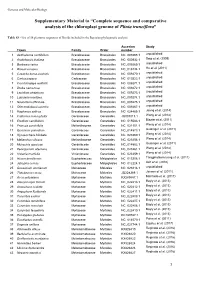
Complete Sequence and Comparative Analysis of the Chloroplast Genome of Plinia Trunciflora”
Genetics and Molecular Biology Supplementary Material to “Complete sequence and comparative analysis of the chloroplast genome of Plinia trunciflora” Table S3 - List of 56 plastome sequences of Rosids included in the Bayesian phylogenetic analysis. Accesion Study Taxon Family Order number 1 Aethionema cordifolium Brassicaceae Brassicales NC_009265.1 unpublished 2 Arabidopsis thaliana Brassicaceae Brassicales NC_000932.1 Sato et al. (1999) 3 Barbarea verna Brassicaceae Brassicales NC_009269.1 unpublished 4 Brassica napus Brassicaceae Brassicales NC_016734.1 Hu et al. (2011) 5 Capsella bursa-pastoris Brassicaceae Brassicales NC_009270.1 unpublished 6 Carica papaya Caricaceae Brassicales NC_010323.1 unpublished 7 Crucihimalaya wallichii Brassicaceae Brassicales NC_009271.1 unpublished 8 Draba nemorosa Brassicaceae Brassicales NC_009272.1 unpublished 9 Lepidium virginicum Brassicaceae Brassicales NC_009273.1 unpublished 10 Lobularia maritima Brassicaceae Brassicales NC_009274.1 unpublished 11 Nasturtium officinale Brassicaceae Brassicales NC_009275.1 unpublished 12 Olimarabidopsis pumila Brassicaceae Brassicales NC_009267.1 unpublished 13 Raphanus sativus Brassicaceae Brassicales NC_024469.1 Jeong et al. (2014) 14 California macrophylla Geraniaceae Geraniales JQ031013.1 Weng et al. (2014) 15 Erodium carvifolium Geraniaceae Geraniales NC_015083.1 Blazier et al. (2011) 16 Francoa sonchifolia Melianthaceae Geraniales NC_021101.1 Weng et al. (2014) 17 Geranium palmatum Geraniaceae Geraniales NC_014573.1 Guisinger et al. (2011) 18 Hypseocharis bilobate Geraniaceae Geraniales NC_023260.1 Weng et al. (2014) 19 Melianthus villosus Melianthaceae Geraniales NC_023256.1 Weng et al. (2014) 20 Monsonia speciose Geraniaceae Geraniales NC_014582.1 Guisinger et al. (2011) 21 Pelargonium alternans Geraniaceae Geraniales NC_023261.1 Weng et al. (2014) 22 Viviania marifolia Vivianiaceae Geraniales NC_023259.1 Weng et al. (2014) 23 Hevea brasiliensis Euphorbiaceae Malpighiales NC_015308.1 Tangphatsornruang et al. -

Checklist of the Vascular Plants of San Diego County 5Th Edition
cHeckliSt of tHe vaScUlaR PlaNtS of SaN DieGo coUNty 5th edition Pinus torreyana subsp. torreyana Downingia concolor var. brevior Thermopsis californica var. semota Pogogyne abramsii Hulsea californica Cylindropuntia fosbergii Dudleya brevifolia Chorizanthe orcuttiana Astragalus deanei by Jon P. Rebman and Michael G. Simpson San Diego Natural History Museum and San Diego State University examples of checklist taxa: SPecieS SPecieS iNfRaSPecieS iNfRaSPecieS NaMe aUtHoR RaNk & NaMe aUtHoR Eriodictyon trichocalyx A. Heller var. lanatum (Brand) Jepson {SD 135251} [E. t. subsp. l. (Brand) Munz] Hairy yerba Santa SyNoNyM SyMBol foR NoN-NATIVE, NATURaliZeD PlaNt *Erodium cicutarium (L.) Aiton {SD 122398} red-Stem Filaree/StorkSbill HeRBaRiUM SPeciMeN coMMoN DocUMeNTATION NaMe SyMBol foR PlaNt Not liSteD iN THE JEPSON MANUAL †Rhus aromatica Aiton var. simplicifolia (Greene) Conquist {SD 118139} Single-leaF SkunkbruSH SyMBol foR StRict eNDeMic TO SaN DieGo coUNty §§Dudleya brevifolia (Moran) Moran {SD 130030} SHort-leaF dudleya [D. blochmaniae (Eastw.) Moran subsp. brevifolia Moran] 1B.1 S1.1 G2t1 ce SyMBol foR NeaR eNDeMic TO SaN DieGo coUNty §Nolina interrata Gentry {SD 79876} deHeSa nolina 1B.1 S2 G2 ce eNviRoNMeNTAL liStiNG SyMBol foR MiSiDeNtifieD PlaNt, Not occURRiNG iN coUNty (Note: this symbol used in appendix 1 only.) ?Cirsium brevistylum Cronq. indian tHiStle i checklist of the vascular plants of san Diego county 5th edition by Jon p. rebman and Michael g. simpson san Diego natural history Museum and san Diego state university publication of: san Diego natural history Museum san Diego, california ii Copyright © 2014 by Jon P. Rebman and Michael G. Simpson Fifth edition 2014. isBn 0-918969-08-5 Copyright © 2006 by Jon P. -

SUPPLEMENTAL TABLE 1 Oenothera Strains of The
RAUWOLF et al. 2008, SUPPLEMENTAL TABLE 1, page 1 of 11 SUPPLEMENTAL TABLE 1 1) Oenothera strains of the subsections Oenothera and Munzia used in RAUWOLF et al. 2008 Genetic Reference for complex Strain Reference for plastome Renner complex (α·β) Diakinesis Strain first described constitution (α and β) Oenothera elata subsp. elata h chapultepec AA-I STUBBE (1963) chapultepec 7 prs. STEINER (1951) STEINER (1951) h cholula AA-I STUBBE (1963) cholula 7 prs. STEINER (1955) STEINER (1955) h puebla AA-I STUBBE (1963) puebla 7 prs. STEINER (1955) STEINER (1955) h toluca AA-I STUBBE (1963) toluca 7 prs. STEINER (1951) STEINER (1951) Oenothera elata subsp. hookeri 2) h franciscana de Vries AA-I STUBBE (1959); franciscana de Vries 7 prs. CLELAND (1935) DAVIS (1916); STINSON (1960) RENNER (1941) 2) h franciscana E. & S. AA-I STUBBE (1959) franciscana E. & S. 7 prs. CLELAND (1935) DAVIS (1916); RENNER (1941) h CLELAND and BLAKESLEE hookeri de Vries AA-I STUBBE (1959) hookeri de Vries 7 prs. DE VRIES (1913) (1931) h johansen AA-I STINSON (1960) johansen 7 prs. CLELAND (1935) CLELAND (1935) Oenothera villosa subsp. villosa 3) St St bauri Standard AA-I STUBBE 1959 laxans· undans 14 BAERECKE (1944) RENNER (1937) RAUWOLF et al. 2008, SUPPLEMENTAL TABLE 1, page 2 of 11 SUPPLEMENTAL TABLE 1 (continued) Genetic Reference for complex Strain Reference for plastome Renner complex (α·β) Diakinesis Strain first described constitution (α and β) Oenothera biennis 4, 5) dV dV biennis de Vries AB-II DE VRIES (1913); albicans· rubens 8, 6 CATCHESIDE (1940) DE VRIES (1901-1903); RENNER (1924) DE VRIES (1913) 4, 5) biM biM biennis München AB-II STUBBE (1959) albicans· rubens 8, 6 CATCHESIDE (1940) RENNER (1917) Col Col chicaginensis Colmar BA-III STUBBE (1963) excellens· punctulans 12, 1 pr. -

Based on Complete Chloroplast Genome Sequences: Effects of Taxon Sampling and Phylogenetic Methods on Resolving Relationships Among Rosids Robert K
Clemson University TigerPrints Publications Genetics and Biochemistry 4-2006 Phylogenetic analyses of Vitis (Vitaceae) based on complete chloroplast genome sequences: effects of taxon sampling and phylogenetic methods on resolving relationships among rosids Robert K. Jansen University of Texas at Austin Charalambos Kaittanis University of Central Florida Christopher Saski Clemson University, [email protected] Seung-Bum Lee University of Central Florida Jeffrey Tomkins Clemson University See next page for additional authors Follow this and additional works at: https://tigerprints.clemson.edu/gen_biochem_pubs Part of the Genetics and Genomics Commons Recommended Citation Please use publisher's recommended citation. This Article is brought to you for free and open access by the Genetics and Biochemistry at TigerPrints. It has been accepted for inclusion in Publications by an authorized administrator of TigerPrints. For more information, please contact [email protected]. Authors Robert K. Jansen, Charalambos Kaittanis, Christopher Saski, Seung-Bum Lee, Jeffrey Tomkins, Andrew Alverson, and Henry Daniell This article is available at TigerPrints: https://tigerprints.clemson.edu/gen_biochem_pubs/3 BMC Evolutionary Biology BioMed Central Research article Open Access Phylogenetic analyses of Vitis (Vitaceae) based on complete chloroplast genome sequences: effects of taxon sampling and phylogenetic methods on resolving relationships among rosids Robert K Jansen1, Charalambos Kaittanis2, Christopher Saski3, Seung- Bum Lee2, Jeffrey Tomkins3, Andrew