Oceanological and Hydrobiological Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Obtaining World Heritage Status and the Impacts of Listing Aa, Bart J.M
University of Groningen Preserving the heritage of humanity? Obtaining world heritage status and the impacts of listing Aa, Bart J.M. van der IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2005 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Aa, B. J. M. V. D. (2005). Preserving the heritage of humanity? Obtaining world heritage status and the impacts of listing. s.n. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 23-09-2021 Appendix 4 World heritage site nominations Listed site in May 2004 (year of rejection, year of listing, possible year of extension of the site) Rejected site and not listed until May 2004 (first year of rejection) Afghanistan Península Valdés (1999) Jam, -
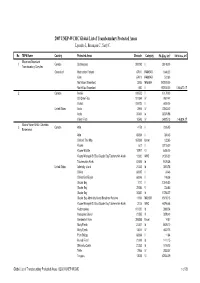
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C
2007 UNEP-WCMC Global List of Transboundary Protected Areas Lysenko I., Besançon C., Savy C. No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Ellesmere/Greenland 1 Canada Quttinirpaaq 300093 II 38148.00 Transboundary Complex Greenland Hochstetter Forland 67910 RAMSAR 1848.20 Kilen 67911 RAMSAR 512.80 North-East Greenland 2065 MAB-BR 972000.00 North-East Greenland 650 II 972000.00 1,008,470.17 2 Canada Ivvavik 100672 II 10170.00 Old Crow Flats 101594 IV 7697.47 Vuntut 100673 II 4400.00 United States Arctic 2904 IV 72843.42 Arctic 35361 Ia 32374.98 Yukon Flats 10543 IV 34925.13 146,824.27 Alaska-Yukon-British Columbia 3 Canada Atlin 4178 II 2326.95 Borderlands Atlin 65094 II 384.45 Chilkoot Trail Nhp 167269 Unset 122.65 Kluane 612 II 22015.00 Kluane Wildlife 18707 VI 6450.00 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 12200 WHC 31595.00 Tatshenshini-Alsek 67406 Ib 9470.26 United States Admiralty Island 21243 Ib 3803.76 Chilkat 68395 II 24.46 Chilkat Bald Eagle 68396 II 198.38 Glacier Bay 1010 II 13045.50 Glacier Bay 22485 V 233.85 Glacier Bay 35382 Ib 10784.27 Glacier Bay-Admiralty Island Biosphere Reserve 11591 MAB-BR 15150.15 Kluane/Wrangell-St Elias/Glacier Bay/Tatshenshini-Alsek 2018 WHC 66796.48 Kootznoowoo 101220 Ib 3868.24 Malaspina Glacier 21555 III 3878.40 Mendenhall River 306286 Unset 14.57 Misty Fiords 21247 Ib 8675.10 Misty Fjords 13041 IV 4622.75 Point Bridge 68394 II 11.64 Russell Fiord 21249 Ib 1411.15 Stikine-LeConte 21252 Ib 1816.75 Tetlin 2956 IV 2833.07 Tongass 13038 VI 67404.09 Global List of Transboundary Protected Areas ©2007 UNEP-WCMC 1 of 78 No TBPA Name Country Protected Areas Sitecode Category PA Size, km 2 TBPA Area, km 2 Tracy Arm-Fords Terror 21254 Ib 2643.43 Wrangell-St Elias 1005 II 33820.14 Wrangell-St Elias 35387 Ib 36740.24 Wrangell-St. -

Komandorsky Zapovednik: Strengthening Community Reserve Relations on the Commander Islands
No. 36 Summer 2004 Special issue: Russia’s Marine Protected Areas PROMOTING BIODIVERSITY CONSERVATION IN RUSSIA AND THROUGHOUT NORTHERN EURASIA CONTENTS CONTENTS Voice from the Wild (A letter from the editors)......................................1 Komandorsky Zapovednik: Strengthening Community Reserve Relations on the Commander Islands......................................24 AN INTRODUCTION TO MARINE Lazovsky Zapovednik: PROTECTED AREAS Working to Create a Marine Buffer Zone...................................................28 MPAs: An Important Tool in Marine Conservation......…………………...2 Kurshskaya Kosa National Park: Tides of Change: Tracing the Development Preserving World Heritage on the Baltic Sea ..........................................30 of Marine Protected Areas in Russia .................................................................4 Dalnevostochny Morskoi Zapovednik: How Effective Are Our MPAs? Looking for Answers An Important Role to Play.........................................................................................6 with Russia’s First Marine Protected Area..................................................32 The Challenges that Lie Ahead.....................………………………………………………8 Russia’s Marine Biosphere Reserves......………………………………………………10 MPA Workshop Offers Opportunities for Dialogue..........................13 THE FUTURE Plans for the Future: Developing a Network of Marine Protected Areas .....................................................……....………………...35 CASE STUDIES An Introduction .............................................................................……....………………...14 -

To View Online Click Here
YOUR O.A.T. ADVENTURE TRAVEL PLANNING GUIDE® The Baltic Capitals & St. Petersburg 2022 Small Groups: 8-16 travelers—guaranteed! (average of 13) Overseas Adventure Travel ® The Leader in Personalized Small Group Adventures on the Road Less Traveled 1 Dear Traveler, At last, the world is opening up again for curious travel lovers like you and me. And the O.A.T. Enhanced! The Baltic Capitals & St. Petersburg itinerary you’ve expressed interest in will be a wonderful way to resume the discoveries that bring us so much joy. You might soon be enjoying standout moments like these: What I love about the little town of Harmi, Estonia, is that it has a lot of heart. Its residents came together to save their local school, and now it’s a thriving hub for community events. Harmi is a new partner of our Grand Circle Foundation, and you’ll live a Day in the Life here, visiting the school and a family farm, and sharing a farm-to-table lunch with our hosts. I love the outdoors and I love art, so my walk in the woods with O.A.T. Trip Experience Leader Inese turned into something extraordinary when she led me along the path called the “Witches Hill” in Lithuania. It’s populated by 80 wooden sculptures of witches, faeries, and spirits that derive from old pagan beliefs. You’ll go there, too (and I bet you’ll be as surprised as I was to learn how prevalent those pagan practices still are.) I was also surprised—and saddened—to learn how terribly the Baltic people were persecuted during the Soviet era. -

Philatelic Market in Russia
Federal State Unitary Enterprise “Marka” Publishing and Trading Center Philatelic Market in Russia June 2016 Philatelic Products Offered by FSUE PTC “Marka” Artistic postage stamps, Catalogs of state signs of souvenir sheets, miniature postal payment sheets First day covers with “Philately” magazine, cancellation Appendix to the magazine Presentation packs with stamps, booklets Artistic stamped envelopes, postcards and envelopes with commemorative stamps and Foreign postage stamps cancellation; maximum cards State Signs of Postal Payment Sales of State Signs of Postal Payment comprises 82% of total revenue. State signs of postal payment are postage stamps and other insignia used to mark mail in order to pay for postal communication services rendered by communication offices according to the existing rates. Signs of postal payment of the Russian Federation must adhere to the UPU Charter and decisions made by the UPU bodies. Standard Stamps Artistic (Commemorative) Stamps and Souvenir Sheets with Letters (“А”, “D”) Envelopes with an Original Stamp State Signs of Reply Domestic Mail Postal Payment Postcards with Letter (“B”) with an Original Stamp Franking Reply Domestic Mail Interaction Pattern The existing system of manufacturing and distribution of state signs of postal payment has been developed for several centuries - development, manufacturing and distribution are separated, which allows to attain high quality of signs of postal payment produced and to eliminate a possibility of abuse. 1 3 FSUE PTC “Marka” FSUE “Russian Post” 2 Delivery of Domestic and Planning, Issuance and Dispatch of International Mails Distribution of State Signs of Marked with State Signs of Postal Postal Payment Payment Export and Import 1. Receipt of order from FSUE “Russian Post” 2. -

2018 FIFA WORLD CUP RUSSIA'n' WATERWAYS
- The 2018 FIFA World Cup will be the 21st FIFA World Cup, a quadrennial international football tournament contested by the men's national teams of the member associations of FIFA. It is scheduled to take place in Russia from 14 June to 15 July 2018,[2] 2018 FIFA WORLD CUP RUSSIA’n’WATERWAYS after the country was awarded the hosting rights on 2 December 2010. This will be the rst World Cup held in Europe since 2006; all but one of the stadium venues are in European Russia, west of the Ural Mountains to keep travel time manageable. - The nal tournament will involve 32 national teams, which include 31 teams determined through qualifying competitions and Routes from the Five Seas 14 June - 15 July 2018 the automatically quali ed host team. A total of 64 matches will be played in 12 venues located in 11 cities. The nal will take place on 15 July in Moscow at the Luzhniki Stadium. - The general visa policy of Russia will not apply to the World Cup participants and fans, who will be able to visit Russia without a visa right before and during the competition regardless of their citizenship [https://en.wikipedia.org/wiki/2018_FIFA_World_Cup]. IDWWS SECTION: Rybinsk – Moscow (433 km) Barents Sea WATERWAYS: Volga River, Rybinskoye, Ughlichskoye, Ivan’kovskoye Reservoirs, Moscow Electronic Navigation Charts for Russian Inland Waterways (RIWW) Canal, Ikshinskoye, Pestovskoye, Klyaz’minskoye Reservoirs, Moskva River 600 MOSCOW Luzhniki Arena Stadium (81.000), Spartak Arena Stadium (45.000) White Sea Finland Belomorsk [White Sea] Belomorsk – Petrozavodsk (402 km) Historic towns: Rybinsk, Ughlich, Kimry, Dubna, Dmitrov Baltic Sea Lock 13,2 White Sea – Baltic Canal, Onega Lake Small rivers: Medveditsa, Dubna, Yukhot’, Nerl’, Kimrka, 3 Helsinki 8 4,0 Shosha, Mologa, Sutka 400 402 Arkhangel’sk Towns: Seghezha, Medvezh’yegorsk, Povenets Lock 12,2 Vyborg Lakes: Vygozero, Segozero, Volozero (>60.000 lakes) 4 19 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 30 1 2 3 6 7 10 14 15 4,0 MOSCOW, Group stage 1/8 1/4 1/2 3 1 Estonia Petrozavodsk IDWWS SECTION: [Baltic Sea] St. -
Pskov from Wikipedia, the Free Encyclopedia Coordinates: 57°49′N 28°20′E
Create account Log in Article Talk Read Edit View history Pskov From Wikipedia, the free encyclopedia Coordinates: 57°49′N 28°20′E Pskov (Russian: Псков; IPA: [pskof] ( listen), ancient Russian spelling "Плѣсковъ", Pleskov) is Navigation Pskov (English) a city and the administrative center of Pskov Oblast, Russia, located about 20 kilometers Псков (Russian) Main page (12 mi) east from the Estonian border, on the Velikaya River. Population: 203,279 (2010 [1] Contents Census);[3] 202,780 (2002 Census);[5] 203,789 (1989 Census).[6] - City - Featured content Current events Contents Random article 1 History Donate to Wikipedia 1.1 Early history 1.2 Pskov Republic 1.3 Modern history Interaction 2 Administrative and municipal status Help 3 Landmarks and sights About Wikipedia 4 Climate Community portal 5 Economy Recent changes 6 Notable people Krom (or Kremlin) in Pskov Contact Wikipedia 7 International relations 7.1 Twin towns and sister cities Toolbox 8 References 8.1 Notes What links here 8.2 Sources Related changes 9 External links Upload file Special pages History [edit] Location of Pskov Oblast in Russia Permanent link Page information Data item Early history [edit] Cite this page The name of the city, originally spelled "Pleskov", may be loosely translated as "[the town] of purling waters". Its earliest mention comes in 903, which records that Igor of Kiev married a [citation needed] Print/export local lady, St. Olga. Pskovians sometimes take this year as the city's foundation date, and in 2003 a great jubilee took place to celebrate Pskov's 1,100th anniversary. Create a book Pskov The first prince of Pskov was Vladimir the Great's younger son Sudislav. -

Woody and Grassy Vegetation Development in Different Landscape Elements of the Curonian Spit
[Type text] Aplinkos tyrimai, inžinerija ir vadyba, 2009. Nr. 4(50), P. 30 - 36 ISSN 1392-1649 Environmental Research, Engineering and Management, 2009. No. 4(50), P. 30 - 36 Woody and Grassy Vegetation Development in Different Landscape Elements of the Curonian Spit Algimantas M. Olšauskas Klaipeda University (received in July, 2009, accepted in December, 2009) The species of woody and grassy vegetation grow on the seashore sands and wastes. These plants are adapted for less favorable existence conditions, some of them growing in littoral habitats of excessive moisture and salinity, the others tolerate infertile and dry sand. The aim of this study has been to analyze the dispersion of vegetation in different relief elements of the coastal protective dunes, to search for relation between woody and grassy plant species, and to foresee the tendencies of further seashore landscape development. It has been established that in the locations of an intensive flow of visitors a net of trodden paths is formed where the plants cover is disappearing very fast as there are suitable conditions for the spring and autumn winds to erode the coastal protective dune of the seashore of the Curonian spit. In a couple of years a trodden path turns into sand drifting corridors of 2 – 3 m. wide, and the trodden and lain places by holiday makers extend to 4 – 5 m. wide pits and hollows. After interconnection of these formations they shape different size deflations. The drifting sand carried by the prevailing western direction winds swamp the plains beyond coastal dunes and the outskirts of the forest and sandy meadows. -

Curonian Spit (Lithuania/Russia) Rehabilitation of Natural Systems of the Spit That Had Been Lost
spiritual aspects, but also to the experience accumulated by generations of local inhabitants, which has permitted the Curonian Spit (Lithuania/Russia) rehabilitation of natural systems of the Spit that had been lost. Criteria ii, iv, and v No 994 Category of property In terms of the categories of cultural property set out in Article 1 of the 1972 World Heritage Convention, this nomination comprises groups of buildings and sites. It is also Identification a cultural landscape as defined in paragraph 39 of the Operational Guidelines for the Implementation of the World Nomination The Curonian Spit Heritage Convention. Location Klaipeda Region, Neringa and Klaipeda (Lithuania); Kaliningrad Region, History and Description Zelenogradsk District (Russian History Federation) Formation of the Curonian Spit began some 5000 years ago. States Party Lithuania and the Russian Federation Despite the continual shifting of its sand dunes, Mesolithic people whose main source of food was from the sea settled Date 23 July 1999 there in the 4th millennium BCE, working bone and stone brought from the mainland. In the 1st millennium CE West Baltic tribes (Curonians and Prussians) established seasonal settlements there, to collect stores of fish, and perhaps also for ritual purposes. Justification by State Party The temperature increase in Europe during the 9th and 10th centuries resulted in a rise of sea level and the creation of the [Note This property is nominated as a mixed site, under the Brockist strait at the base of the Spit. This provided the basis natural and the cultural criteria. This evaluation will deal for the establishment of the pagan trading centre of Kaup, solely with the cultural values, and the natural values will be which flourished between c 800 and 1016. -

Evolution of the Curonian Spit Dunes
KLAIPĖDA UNIVERSITY Nikita DOBROTIN EVOLUTION OF THE CURONIAN SPIT DUNES DOCTORAL DISSERTATION Biomedical sciences, ecology and environmental sciences (03B) Klaipėda, 2018 Doctoral dissertation was prepared during the period 2011–2018 at Klaipėda Univer- sity, based on the conferment a doctorate right which was granted for Klaipėda Uni- versity by the order of the Minister of Education and Science (Republic of Lithuania) No. V-574, signed on 17th July, 2017. Academic advisor prof. dr. Albertas BITINAS (Klaipėda University, Physical Sciences, Geology – 05P) The doctoral dissertation will be defended at the Board of Klaipėda University in Ecology and Environmental Sciences: Chairman prof. dr. Zita Rasuolė GASIŪNAITĖ (Klaipėda University, Biomedical Sciences, Ecology and Environmental Sciences – 03B) Members: dr. Nerijus BLAŽAUSKAS (Klaipėda University, Physical Sciences, Geology Sciences – 05P), doc. dr. Martynas BUČAS (Klaipėda University, Biomedical Sciences, Ecology and Environmental Sciences – 03B), dr. Alar ROSENTAU (Tartu University, Estonia, Physical Sciences, Geology Sci- ences – 05P), prof. habil. dr. Sergej OLENIN (Klaipėda University, Biomedical Sciences, Ecol- ogy and Environmental Sciences – 03B). The doctoral dissertation will be defended in a public meeting of the Board in Ecology and Environmental Sciences, Klaipėda University, Aula Magna Conference hall at 1 p.m. on 19th of October, 2018. Address: Herkaus Manto str. 84, LT-92294, Klaipėda, Lithuania. The doctoral dissertation was sent out on 19th of September 2018. The doctoral dissertation is available for review at the Library of the Klaipėda University. KLAIPĖDOS UNIVERSITETAS Nikita DOBROTIN KURŠIŲ NERIJOS KOPŲ RAIDA DAKTARO DISERTACIJA Biomedicinos mokslai, ekologija ir aplinkotyra (03B) Klaipėda, 2018 Disertacija rengta 2011–2018 metais Klaipėdos universitete pagal suteiktą Klaipėdos universitetui Lietuvos Respublikos švietimo ir mokslo ministro 2017 m. -

The Ant-Like Flowerbeetles (Coleoptera: Anthicidae) of the Curonian Spit (Lithuania)
NAUJOS IR RETOS LIETUVOS VABZDŽI Ų R ŪŠYS. 22 tomas 17 THE ANT-LIKE FLOWERBEETLES (COLEOPTERA: ANTHICIDAE) OF THE CURONIAN SPIT (LITHUANIA) ROMAS FERENCA ¹ ², POVILAS IVINSKIS ¹, JOLANTA RIMŠAIT Ė ¹ ¹ Nature Research Centre, Institute of Ecology, Akademijos 2, LT-08412 Vilnius, Lithuania E-mail: [email protected], [email protected] ²Kaunas T. Ivanauskas Zoological Museum, Laisv ės. 106, LT-44253 Kaunas, Lithuania E-mail: [email protected], [email protected] Abstract. Beetles of eight species: Anthicus antherinus L., A. ater Panz., A. bimaculatus Ill., A. flavipes Panz., A. sellatus Panz., Cordicomus gracilis Panz., Omonadus formicarius Goeze and Notoxus monoceros L. have been found during our research in the Curonian Spit. Cordicomus gracilis Panz and Omonadus formicarius Goeze are very rare in Lithuania; only single specimens of this species were found on the shore of the Curonian Lagoon during our research. Key words: Anthicidae, Curonian Spit, Lithuania Introduction The most important information on the fauna of Coleoptera of the Curonian Spit was summarized in monographs on Lithuanian beetles (Pileckis, 1976; Pileckis & Monsevi čius, 1995, 1997). Since the appearance of these publications a lot of time has passed, and new data on the Coleoptera of Curonian Spit were published by some other authors (Ferenca 2003, 2004, 2006; Ferenca & Tamutis, 2009; Ferenca et al . 2002, 2006, 2007; Ivinskis et al . 2003, 2009; Ivinskis & Rimšait ė 2005; Šablevi čius 2003, 2004; Tamutis & Ferenca 2006; Tamutis et. al ., 2008) during the period of 2002–2009. Ant- like flowerbeetles (Coleoptera: Anthicidae) are phytosaprophagous, their larvae develop in decayed vegetation (Pileckis &. Monsevi čius, 1997). According to the published data, the fauna of Lithuanian ant-like flowerbeetles comprises a total of 12 species (Pileckis & Monsevi čus, 1997; Barševskis, 2001; Tamutis, 2003). -

Anniversary of the Inscription of First Russian Sites on the World Heritage List Ministry of Culture of the Russian Federation
Ministry of Culture of the Russian Federation Anniversary of the Inscription of First Russian Sites on the World Heritage List Ministry of Culture of the Russian Federation Russian World Heritage Sites – 16 cultural, 10 natural properties 1990 – 2014 1988 1990 1992 1993 1994 1995 1996 1998 1999 2000 2001 2003 2004 2005 2010 2012 2014 Ministry of Culture of the Russian Federation 2000 Historic and Architectural Complex of the Kazan Kremlin 2014 Bolgar Historic and Archeological Complex Ministry of Culture of the Russian Federation Saint Peters burg Leningradskaya oblast Ministry of Culture of the Russian Federation Kremlin and Red Square, Moscow Ministry of Culture of the Russian Federation Kizhi Pogost, Karelia Ministry of Culture of the Russian Federation Ministry of Culture of the Russian Federation State Protection: zoning, restrictions, control and supervision Ministry of Culture of the Russian Federation Preservation Ministry of Culture of the Russian Federation Legislation The President of the Russian Federation The Federal Assembly Management of the Russian Plan, Heritage Federation Impact Assessment, Buffer Zone Ministry of Culture of the Russian Federation Ministry of Culture of the Russian Federation Coordination Russian versions on http://mkrf. ru/ Ministry of Culture of the Russian Federation 14-16 December 2015 Anniversary exhibition: Year of Literature in Russia learn more UNESCO 70 about the First Russian Sites on the World Heritage List Welcome to Saint-Petersburg International Cultural Forum 14-16 December 2015! Saint Petersburg Administration 39th Session of the World Heritage Committee, Bonn, 3 July 2015 Photo by A.Pashkevich 3 July 2015 Address by H.E. Ms Eleonora Mitrofanova, on the occasion of the 25th anniversary of first Russian cultural monuments’ inscriptions in UNESCO World Heritage List Photo©Russian Delegation to UNESCO: Konstantin VOLKOV Dear members of the World Heritage Committee, Dear representatives of States Parties and advisory bodies, Ladies and Gentlemen.