Answer Key Chapter 4: Standard Review Worksheet 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report of the Advisory Group to Recommend Priorities for the IARC Monographs During 2020–2024
IARC Monographs on the Identification of Carcinogenic Hazards to Humans Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 Report of the Advisory Group to Recommend Priorities for the IARC Monographs during 2020–2024 CONTENTS Introduction ................................................................................................................................... 1 Acetaldehyde (CAS No. 75-07-0) ................................................................................................. 3 Acrolein (CAS No. 107-02-8) ....................................................................................................... 4 Acrylamide (CAS No. 79-06-1) .................................................................................................... 5 Acrylonitrile (CAS No. 107-13-1) ................................................................................................ 6 Aflatoxins (CAS No. 1402-68-2) .................................................................................................. 8 Air pollutants and underlying mechanisms for breast cancer ....................................................... 9 Airborne gram-negative bacterial endotoxins ............................................................................. 10 Alachlor (chloroacetanilide herbicide) (CAS No. 15972-60-8) .................................................. 10 Aluminium (CAS No. 7429-90-5) .............................................................................................. 11 -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

Potassium Bromate
POTASSIUM BROMATE VWR International, Pty Ltd Chemwatch: 1484 Issue Date: 25/01/2013 Version No: 6.1.1.1 Print Date: 10/12/2013 Safety Data Sheet according to WHS and ADG requirements S.GHS.AUS.EN SECTION 1 IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING Product Identifier Product name POTASSIUM BROMATE Chemical Name potassium bromate Synonyms Br-K-O3, KBrO3, bromic acid potassium salt Proper shipping name POTASSIUM BROMATE Chemical formula BrHO3.K Other means of identification Not Available CAS number 7758-01-2 Relevant identified uses of the substance or mixture and uses advised against Used as laboratory reagent, oxidising agent, permanent wave compound, maturing agent in flour, dough conditioner and food additive. Bromate is Relevant identified uses converted to bromide in the baking or cooking process, but the levels are not in excess of the natural bromide content of many natural foods., Note: Food additive uses restricted as to proportions used., [~Intermediate ~] Details of the supplier of the safety data sheet Registered company name VWR International, Pty Ltd Unit 1/31 Archimedes Place 4172 QLD Address Australia Telephone 61 7 3009 4100 ; 1300 727 696 Fax 61 7 3009 4199 ; 1300 135 123 Website http://au.vwr.com Email [email protected] Emergency telephone number Association / Organisation Not Available Emergency telephone numbers 61 7 3009 4100 ; 1300 727 696 Other emergency telephone numbers 61 7 3009 4100 ; 1300 727 696 SECTION 2 HAZARDS IDENTIFICATION Classification of the substance or mixture HAZARDOUS CHEMICAL. DANGEROUS GOODS. According to the Model WHS Regulations and the ADG Code. -

Abstract Measurement and Analysis of Bromate Ion
ABSTRACT MEASUREMENT AND ANALYSIS OF BROMATE ION REDUCTION IN SYNTHETIC GASTRIC JUICE by Jason Dimitrius Keith Bromate ion is a possible carcinogen that is regulated by the US EPA at a Maximum Contamination Level (MCL) of 10 µg/L in drinking water. In order to propose an improved scientifically appropriate bromate ion MCL, a more rigorous scientific methodology is needed for determining low level dose health risks. The objectives of this research project were to measure bromate ion with oxidizing and/or reducing agents typically ingested in foods and drinking water. The loss of bromate ion in HCl is too slow for significant reduction in the stomach. -5 Addition of 10 M H2S, a gastric juice component, decreases the half-life from 153 to 14 minutes. The ingested reducing agents iodide ion, nitrite ion, and iron(II) decrease the lifetime of bromate ion in the stomach. Chlorine, monochloramine, and iron(III) have little actual effect on the lifetime of bromate ion. The measured rates and chemical details of the reactions are discussed. MEASUREMENT AND ANALYSIS OF BROMATE ION REDUCTION IN SYNTHETIC GASTRIC JUICE A Thesis Submitted to the faculty of Miami University in partial fulfillment of the requirements for the degree of Master of Science Department of Chemistry by Jason Dimitrius Keith Miami University Oxford, Ohio 2005 Co-Advisor________________ (Dr. Gilbert Gordon) Co-Advisor________________ (Dr. Gilbert E. Pacey) Reader_________________ (Dr. Michael W. Crowder) Reader_________________ (Dr. Hongcai Zhou) TABLE OF CONTENTS TABLE OF CONTENTS ii LIST OF TABLES iii LIST OF FIGURES iv ACKNOWLEDGEMENTS v INTRODUCTION 1 Bromate Ion Chemistry and Human Toxicology 1 Prior Analytical Methodology 6 Objectives 7 METHOD DEVELOPMENT AND ESTABLISHMENT OF PROTOCOLS 7 Solution Preparation 7 Preparation and Measurement of Stock HOCl/ Cl2 and ClNH2 Solutions 11 Measurement of Iron(II) and Iron(III) in Solution 12 Instrumentation. -
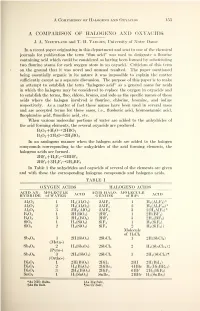
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Bromate in Sodium Hypochlorite--Potable Water Treatment General
THE CHLORINE INSTITUTE, INC. Bromate in Sodium Hypochlorite--Potable Water Treatment General On December 16, 2001,Stage I of the Disinfectants / Disinfection Byproducts Rule will require potable water plants to meet a bromate M.C.L. of 10 parts per billion (ppb) in their effluents. Plants that use ozone in their treatment process will be required to test monthly for bromate. Plants that do not use ozone, but use sodium hypochlorite solutions will not need to test, they will be protected by certification to ANSI / NSF Standard # 60 and/or the AWWA Standard for Hypochlorites. Industry is working with both organizations to develop specifications that easily meet this M.C.L. The sodium hypochlorite Industry wants to provide a realistic safety margin so that testing for bromate will not be required in potable water treated with this chemical. How did Bromates Get into Sodium Hypochlorite & Can they be Removed? Bromide ions are found in the salt used to make both chlorine and sodium hydroxide, the two raw materials reacted to form sodium hypochlorite. Virtually all of the bromine in chlorine and the bromide in the sodium hydroxide quickly becomes bromate at the pH of NaOCl . The concentration of bromide varies tremendously in different salt sources. It also partitions between the two chemicals (chlorine and sodium hydroxide) differently depending on the type of electrochemical cells used in the process. Some plants can change their source of salt, while others are located near salt mines and are limited to the salt they have available. Current technology cannot easily or economically remove bromate or its precursor from either the initial salt, the two reactants or the final sodium hypochlorite solution. -

NACE Bromine Chemistry Review Paper
25 YEARS OF BROMINE CHEMISTRY IN INDUSTRIAL WATER SYSTEMS: A REVIEW Christopher J. Nalepa Albemarle Corporation P.O. Box 14799 Baton Rouge, LA 70898 ABSTRACT Bromine chemistry is used to great advantage in nature for fouling control by a number of sessile marine organisms such as sponges, seaweeds, and bryozoans. Such organisms produce small quantities of brominated organic compounds that effectively help keep their surfaces clean of problem bacteria, fungi, and algae. For over two decades, bromine chemistry has been used to similar advantage in the treatment of industrial water systems. The past several years in particular has seen the development of several diverse bromine product forms – one-drum stabilized bromine liquids, all-bromine hydantoin solids, and pumpable gels. The purpose of this paper is to review the development of bromine chemistry in industrial water treatment, discuss characteristics of the new product forms, and speculate on future developments. Keywords: Oxidizing biocide, bleach, bromine, bromine chemistry, sodium hypobromite, activated sodium bromide, Bromochlorodimethylhydantoin, Bromochloromethyethylhydantoin, Dibromodi- methylhydantoin,, BCDMH, BCMEH, DBDMH, stabilized bromine chloride, stabilized hypobromite INTRODUCTION Sessile marine organisms generate metabolites to ward off predators and deter attachment of potential micro- and macrofoulants. Sponges, algae, and bryozoans for example, produce a rich variety of bromine-containing compounds that exhibit antifoulant properties (Fig. 1).1,2,3 Scientists are actively studying these organisms to understand how they maintain surfaces that are relatively clean and slime- free.4 Brominated furanones isolated from the red algae Delisea pulchra, for example, have been found to interfere with the chemical signals (acylated homoserine lactones) that bacteria use to communicate with one another to produce biofilms.5,6 This work may eventually lead to more effective control of microorganisms in a number of industries such as industrial water treatment, oil and gas production, health care, etc. -

WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/074683 Al 19 May 2016 (19.05.2016) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C12N 15/10 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/DK20 15/050343 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 11 November 2015 ( 11. 1 1.2015) KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (25) Filing Language: English PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (26) Publication Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: PA 2014 00655 11 November 2014 ( 11. 1 1.2014) DK (84) Designated States (unless otherwise indicated, for every 62/077,933 11 November 2014 ( 11. 11.2014) US kind of regional protection available): ARIPO (BW, GH, 62/202,3 18 7 August 2015 (07.08.2015) US GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, (71) Applicant: LUNDORF PEDERSEN MATERIALS APS TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, [DK/DK]; Nordvej 16 B, Himmelev, DK-4000 Roskilde DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, (DK). -

CHEM 1411 Nomenclature Homework - Answers Part I
1 CHEM 1411 Nomenclature Homework - Answers Part I 1. The following are a list of binary and pseudobinary ionic compounds. Write the name when the formula is given. Write the formula when the name is given. (a) AlCl3 aluminum chloride (k) rubidium oxide Rb2O (b) AuBr3 gold (III) bromide (l) chromium (III) selenide Cr2Se3 (c) Na2S sodium sulfide (m) barium iodide BaI2 (d) Cu3P2 copper (II) phosphide (n) copper (I) fluoride CuF (e) Fe(OH)2 iron (II) hydroxide (o) copper (II) fluoride CuF2 (f) NH4OH ammonium hydroxide (p) strontium cyanide Sr(CN)2 (g) Co(CH3COO)3 cobalt (III) acetate (q) mercury (II) bromide HgBr2 (h) Zn(SCN)2 zinc thiocyanate (r) mercury (I) bromide Hg2Br2 (i) CaCrO4 calcium chromate (s) magnesium permanganate Mg(MnO4)2 (j) K2Cr2O7 potassium dichromate (t) lithium nitride Li3N 2. The following are lists of covalent compounds. Write the name when a formula is given. Write the formula when given a name. (a) CSe2 carbon diselenide (h) dichlorine heptoxide Cl2O7 (b) SF6 sulfur hexafluoride (i) xenon tetrafluoride XeF4 (c) BrF5 bromine pentafluoride (j) carbon monoxide CO (d) P4O10 tetraphosphorous decoxide (k) oxygen O2 (e) Cl2O dichlorine oxide (l) diboron trioxide B2B O3 (f) NH3 ammonia (m) arsenic trifluoride AsF3 (g) N2 dinitrogen or nitrogen (n) diiodine I2 2 3. The following are lists of acids or acid-forming compounds. Write the name when the formula is given. Write the formula when the name is given. (a) H3PO2 hypophosphorous acid (k) hydrogen cyanide HCN (g) (b) H2SO4 sulfuric acid (l) periodic acid HIO4 (c) HClO hypochlorous acid (m) hypochlorous acid HClO (d) H3PO4 phosphoric acid (n) nitric acid HNO3 (e) HBrO4 perbromic acid (o) acetic acid CH3CO2H (f) HIO2 iodous acid (p) chloric acid HClO3 (g) HI (g) hydrogen iodide (q) perbromic acid HBrO4 (h) HI (aq) hydroiodic acid (r) hydrofluoric acid HF (aq) (i) HCN (aq) hydrocyanic acid (s) phosphorous acid H3PO3 (j) HBrO hypobromous acid (t) hydrosulfuric acid H2S (aq) 4. -

WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2013/089962 Al 20 June 2013 (20.06.2013) W P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every B01J 31/04 (2006.01) B01J 31/18 (2006.01) kind of national protection available): AE, AG, AL, AM, B01J 31/14 (2006.01) B01J 31/22 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 12/065285 HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, (22) International Filing Date: KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, 15 November 2012 (15.1 1.2012) ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, (25) Filing Language: English RW, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, (26) Publication Language: English TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: 13/323,328 12 December 201 1 (12. 12.201 1) US (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (71) Applicant (for all designated States except US): CHEV¬ GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, RON PHILLIPS CHEMICAL COMPANY LP UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, [US/US]; 10001 Six Pines Drive, The Woodlands, Texas TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, 77380 (US). -

AP Chemistry Summer Assignment 2021-22 School Year
AP Chemistry AP Chemistry Summer Assignment 2021-22 School Year Chemical Foundations Chapter 1 1. How many significant figures are in the following numbers? a. 0.00150 b. 0.1205 c. 200 d. 2.00 X 10 3 2. Complete the operation and report using the correct number of significant figures a. 26.20 - 0.5= b. 2.5 + 3.25 = c. .040 X 2.0 = d. 3.25 / 4 = e. 3.0 X 10 -3 X 6 = Atomic , Molecules Ions CH 2 and Stoichiometry CH 3 with Periodicity CH 7 3. What is an isotope? Refer to the isotope of Uranium 92U 4. How many protons and neutrons are in the nucleus of this isotope. 5. How many electrons are in a single atom of Uranium 6. What is the mass of this isotope of Uranium. 7. Assume silicon has three major isotopes in nature. The average atomic mass of silicon is 28.09 amu. Fill in the missing information in the table. Isotope Mass (amu) Abundance 28Si 27.89 29Si 4.70% 30Si 29.97 3.09% 8. Which color of light has the highest frequency, red or green ? 9. Which color of light has the longest wavelength, green or violet? 10. Hydrogen emits light with a wavelength of 410 nm, what is the frequency of this light? 11. What is the electron configuration, orbital notation and noble gas notation for phosphorus? For bromine? How many unpaired electrons does phosphorus have? How many unpaired electrons does bromine have? 12. What is the charge for a phosphorus ion? Why does it make this charge? 13.