Comparative Morphology of the Forelimb Digging Apparatus in Armadillos (Xenarthra: Cingulata, Dasypodidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
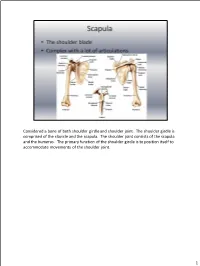
Considered a Bone of Both Shoulder Girdle and Shoulder Joint. the Shoulder Girdle Is Comprised of the Clavicle and the Scapula
Considered a bone of both shoulder girdle and shoulder joint. The shoulder girdle is comprised of the clavicle and the scapula. The shoulder joint consists of the scapula and the humerus. The primary function of the shoulder girdle is to position itself to accommodate movements of the shoulder joint. 1 Superior angle—top point Inferior angle—bottom point Vertebral border—side closest to vertebral column Axillary border—side closest to arm Subscapular fossa—anterior fossa Glenoid fossa, glenoid labrum, glenoid cavity --The glenoid fossa is the shallow cavity where the humeral head goes. The glenoid labrum is the cartilage that goes around the glenoid fossa. So the glenoid fossa and glenoid labrum together comprise the glenoid cavity. Supraspinous fossa—posterior, fossa above the spine Spine of the scapula—the back projection Infraspinous fossa—posterior depression/fossa below spine Coracoid process—anterior projection head Acromion process—posterior projection head above spine 2 Scapulothoracic “joint” = NOT a true joint; there are no ligaments or articular capsule. The scapula just rests on the muscle over top the rib cage, which allows for passive movements. Sternoclavicular joint=where the clavicle (collarbone) and the sternum (breastbone) articulate; movement is slight in all directions and of a gliding, rotational type Acromioclavicular joint = where the clavicle and scapula (acromion process) articulate; AKA: AC Joint; movement is a slight gliding when elevation and depression take place. Glenohumeral joint = the shoulder joint 3 4 All 3 true joints: Sternoclavicular, AC and glenohumeral (GH) all work together to move arm in all directions. The GH allows the arm to go out to the side and be abducted, then the AC and Sternoclavicular joints kick in to allow the arm to go above shoulder level by allowing the shoulderblade to move up to increase the range of motion (ROM). -

Cartilla-Armadillos-Llanos-Orientales
Los Orientales Tabla de contenido Ilustraciones zOOm diseño S.A.S Ma. Cecilia Isaza R. Impresión Unión Gráfica Ltda. 4 Introducción Cítese como: Rodríguez P., Superina M., Edición de textos Cruz-Antia D. & F. Trujillo, 2013. Los Arma- Iván Bernal-Neira 6 ¿Dónde se originaron los armadillos? dillos de los Llanos Orientales de Colombia. Características de los armadillos ODL S.A. - Fundación Omacha - Corporino- ISBN: 978-958-8554-32-7 9 quia - Cormacarena - Corpometa - Bioparque Descripción de las especies de armadillos presentes Los Ocarros. Cartilla realizada en el marco del proyecto 11 en los Llanos Orientales “Conservación y manejo de los armadillos Autores en el área de influencia del Oleoducto de 18 Los armadillos de los Llanos Orientales y sus diferencias Paola Rodríguez, Mariella Superina, Daniel los Llanos Orientales”, ejecutado bajo el 20 ¿Dónde viven los armadillos de los Llanos? Cruz-Antia & Fernando Trujillo. convenio de cooperación establecido en- tre ODL S.A., la Fundación Omacha, Cor- 24 ¿Cuándo y cómo se reproducen los armadillos? Fotografías porinoquia, Cormacarena, Corpometa y el Daniel Cruz-Antia, Fernando Trujillo, Paola Bioparque Los Ocarros. 26 ¿Cómo de alimentan y qué comen los armadillos? Rodríguez, Emilio Constantino, Luis Gabriel ¿Cuál es el valor de los armadillos para los ecosistemas? Amado, Carlos Aya, Jairán Sánchez, Carlos To- 27 rrente, Sindy Martínez, Mogens Trolle y Fede- 29 ¿Por qué podrían desaparecer los armadillos en los Llanos? rico Pardo. 35 Categorías de amenaza para los armadillos Diseño y diagramación Oportunidades de conservación zOOm diseño S.A.S 37 Luisa Fda. Cuervo G. 40 Bibliografía Introducción para la comercialización de su carne y la tenencia como mascotas, el aumento de perros domésti- cos en áreas rurales y los atropellamientos en las Los armadillos son un grupo de mamíferos exclusi- carreteras. -

Distinguishing Quaternary Glyptodontine Cingulates in South America: How Informative Are Juvenile Specimens?
Distinguishing Quaternary glyptodontine cingulates in South America: How informative are juvenile specimens? CARLOS A. LUNA, IGNACIO A. CERDA, ALFREDO E. ZURITA, ROMINA GONZALEZ, M. CECILIA PRIETO, DIMILA MOTHÉ, and LEONARDO S. AVILLA Luna, C.A., Cerda, I.A., Zurita, A.E., Gonzalez, R., Prieto, M.C., Mothé, D., and Avilla, L.S. 2018. Distinguishing Quaternary glyptodontine cingulates in South America: How informative are juvenile specimens? Acta Palaeontologica Polonica 63 (1): 159–170. The subfamily Glyptodontinae (Xenarthra, Cingulata) comprises one of the most frequently recorded glyptodontids in South America. Recently, the North American genus Glyptotherium was recorded in South America, in addition to the genus Glyptodon. It has been shown that both genera shared the same geographic distribution in central-north and eastern areas of South America (Venezuela and Brazil, respectively). Although some characters allow differentiation between adult specimens of both genera, the morphological distinction between these two genera is rather difficult in juvenile specimens. In this contribution, a detailed morphological, morphometric and histological survey of a juvenile specimen of Glyptodontinae recovered from the Late Pleistocene of northern Brazil is performed. The relative lower osteoderms thickness, the particular morphology of the annular and radial sulci and the distal osseous projections of the caudal osteoderms suggest that the specimen belongs to the genus Glyptotherium. In addition, the validity of some statistical tools to distinguish between different ontogenetic stages and in some cases between genera is verified. The osteoderm microstructure of this juvenile individual is characterized by being composed of a cancellous internal core surrounded by a compact bone cortex. Primary bone tissue mostly consists of highly vascularized, woven-fibered bone tissue. -

The Giant Armadillo of the Gran Chaco
The Giant Armadillo of the Gran Chaco A giant armadillo Priodontes maximus at the Saenz-Peña Zoo in South America raises up, balancing with its tail, a common posture for this large species. Venezuela The Guianas: Guyana hat’s the size of Texas and Arizona combined, reaches temperatures Suriname French Guiana Wof 115 degrees Fahrenheit, has plants with 15-inch-long thorns, Colombia and houses an armadillo larger than a coffee table? The South American Gran Chaco, where giant armadillos wander freely. The Gran Chaco region covers more than 1 million square kilometers of Argentina, Bolivia, Perú Brazil Paraguay, and Brazil, with approximately 60 percent in Argentina and Bolivia just 7 percent in Brazil. The region is a mosaic of grasslands, savannas, Paraguay • open woodlands, dry thorn forests, and gallery forests that provide a GRAN CHACO 15 range of habitats where some diverse animal species flourish. • In the gallery forests of the humid Chaco, we regularly encounter animals Argentina that are associated with tropical and subtropical forests, like jaguars, owl monkeys, howler monkeys, peccaries, deer, tapirs, and various kinds of eden- tates, a group of mammals that includes sloths, anteaters, and armadillos. The Gran Chaco—from the Quechua Although there are no sloths in the Chaco, we regularly find lesser anteaters 2003 and sometimes come across giant anteaters. Both the nine-banded armadillo, Indian language of Bolivia for “great hunting ground”—crosses four coun- also found in Texas, and the tatu bola, or three-banded armadillo, which you tries and encompasses an area the can see at the Wild Animal Park’s Animal Care Center and the San Diego Zoo’s size of Texas and Arizona combined. -

El Armadillo, Cabassous Centralis (Cingulata: Chlamyphoridae) En Agroecosistemas Con Café De Costa Rica
El armadillo, Cabassous centralis (Cingulata: Chlamyphoridae) en agroecosistemas con café de Costa Rica Ronald J. Sánchez-Brenes1 & Javier Monge2 1. Universidad Nacional Costa Rica, Sede Regional Chorotega, Centro Mesoamericano de Desarrollo Sostenible del Trópico Seco (CEMEDE-UNA), Nicoya, Costa Rica; [email protected], https://orcid.org/0000-0002-6979-1336 2. Universidad de Costa Rica, Facultad de Ciencias Agroalimentarias, Escuela de Agronomía, Centro de Investigación en Protección de Cultivos, Instituto de Investigaciones Agronómicas, San José, Costa Rica; [email protected], https://orcid.org/0000-0003-1530-5774 Recibido 09-VIII-2019 • Corregido 11-IX-2019 • Aceptado 30-IX-2019 DOI: https://doi.org/10.22458/urj.v11i3.2724 ABSTRACT: “The armadillo, Cabassous centralis (Cingulata: RESUMEN: Introducción: El armadillo Cabassous centralis se clasifica Chlamyphoridae) in a Costa Rican coffee agro-ecosystem”. Introduction: como una especie con Datos Insuficientes que se encuentra desde The rare Cabassous centralis armadillo is classified as a Data Deficient México hasta el norte de América del Sur. Objetivo: Ampliar la distri- species found from Mexico to northern South America). Objective: To bución ecológica de C. centralis. Métodos: Colocamos cuatro cámaras expand the ecological distribution of C. centralis. Methods: We placed trampa en sitios estratégicos como fuentes de alimentación, madrigue- four trap cameras in strategic sites such as food sites, burrows, bodies of ras, cuerpos de agua y transición al bosque secundario, en San Ramón, water and transition to the secondary forest, in San Ramón, Costa Rica. Costa Rica. Resultados: Obtuvimos un registro de C. centralis en la tran- Results: We obtained a record of C. -

(Dasypus) in North America Based on Ancient Mitochondrial DNA
bs_bs_banner A revised evolutionary history of armadillos (Dasypus) in North America based on ancient mitochondrial DNA BETH SHAPIRO, RUSSELL W. GRAHAM AND BRANDON LETTS Shapiro, B. Graham, R. W. & Letts, B.: A revised evolutionary history of armadillos (Dasypus) in North America based on ancient mitochondrial DNA. Boreas. 10.1111/bor.12094. ISSN 0300-9483. The large, beautiful armadillo, Dasypus bellus, first appeared in North America about 2.5 million years ago, and was extinct across its southeastern US range by 11 thousand years ago (ka). Within the last 150 years, the much smaller nine-banded armadillo, D. novemcinctus, has expanded rapidly out of Mexico and colonized much of the former range of the beautiful armadillo. The high degree of morphological similarity between these two species has led to speculation that they might be a single, highly adaptable species with phenotypical responses and geographical range fluctuations resulting from environmental changes. If this is correct, then the biology and tolerance limits for D. novemcinctus could be directly applied to the Pleistocene species, D. bellus. To investigate this, we isolated ancient mitochondrial DNA from late Pleistocene-age specimens of Dasypus from Missouri and Florida. We identified two genetically distinct mitochondrial lineages, which most likely correspond to D. bellus (Missouri) and D. novemcinctus (Florida). Surprisingly, both lineages were isolated from large specimens that were identified previously as D. bellus. Our results suggest that D. novemcinctus, which is currently classified as an invasive species, was already present in central Florida around 10 ka, significantly earlier than previously believed. Beth Shapiro ([email protected]), Department of Ecology and Evolutionary Biology, University of California Santa Cruz, Santa Cruz, CA 95064, USA; Russell W. -

Guidelines to Identify Individual Giant Armadillos, Priodontes Maximus (Kerr, 1792), Through Camera Traps
Edentata 20 (2019): 1–16 DOI: 10.2305/IUCN.CH.2019.Edentata-20-1.2.en Electronic version: ISSN 1852-9208 Print version: ISSN 1413-4411 http://www.xenarthrans.org Guidelines to identify individual giant armadillos, Priodontes maximus (Kerr, 1792), through camera traps Gabriel Fávero MassocatoA,B,D,1 & Arnaud L. J. DesbiezA,C,D A Instituto de Conservação de Animais Silvestres (ICAS), Rua Afonso Lino Barbosa, 142, Chácara Cachoeira, 79040-290, Campo Grande, Mato Grosso do Sul, Brasil B Houston Zoo, 6200 Hermann Park Drive, Houston, Texas 77030, USA C Royal Zoological Society of Scotland (RZSS), Murrayfield, Edinburgh, EH12 6TS, United Kingdom D Instituto de Pesquisas Ecológicas (IPÊ), Rodovia Dom Pedro I, km 47, 12960-000, Nazaré Paulista, São Paulo, Brasil 1 Corresponding author E-mail: [email protected] Abstract Camera trapping is one of the main tools used to advance Priodontes maximus research as it can provide information on the species' presence, densities, relative abundance, home ranges, movement, ac- tivity patterns, habitat use, reproduction, and parental care. Photographic records obtained by camera traps allow the individual identification of P. maximus if properly examined. The aim of this work is to pro- vide researchers with the tools to identify individuals of P. maximus in their regions and stimulate further research and conservation work on the species. We use nine years of camera trap work to present and il- lustrate the different individual identification patterns that can be used to distinguish individuals as well as reproductive status and age class. We describe six different morphological characteristics that can be used for individual identification: cephalic scale pattern, tail markings, light band width and shape above the base of tail, hind limbs, flank scale pattern, and natural marks. -

Nine-Banded Armadillo (Dasypus Novemcinctus) Michael T
Nine-banded Armadillo (Dasypus novemcinctus) Michael T. Mengak Armadillos are present throughout much of Georgia and are considered an urban pest by many people. Armadillos are common in central and southern Georgia and can easily be found in most of Georgia’s 159 counties. They may be absent from the mountain counties but are found northward along the Interstate 75 corridor. They have poorly developed teeth and limited mobility. In fact, armadillos have small, peg-like teeth that are useful for grinding their food but of little value for capturing prey. No other mammal in Georgia has bony skin plates or a “shell”, which makes the armadillo easy to identify. Just like a turtle, the shell is called a carapace. Only one species of armadillo is found in Georgia and the southeastern U.S. However, 20 recognized species are found throughout Central and South America. These include the giant armadillo, which can weigh up to 130 pounds, and the pink fairy armadillo, which weighs less than 4 ounces. Taxonomy Order Cingulata – Armadillos Family Dasypodidae – Armadillo Nine-banded Armadillo – Dasypus novemcinctus The genus name Dasypus is thought to be derived from a Greek word for hare or rabbit. The armadillo is so named because the Aztec word for armadillo meant turtle-rabbit. The species name novemcinctus refers to the nine movable bands on the middle portion of their shell or carapace. Their common name, armadillo, is derived from a Spanish word meaning “little armored one.” Figure 1. Nine-banded Armadillo. Photo by author, 2014. Status Armadillos are considered both an exotic species and a pest. -

Phty 101 – Week 1
PHTY 101 – WEEK 1 1.7 Classify the shoulder (glenohumeral) joint and identify and/or describe its: • The glenohumeral joint is a multiaxial, synovial, ball and socket joint • Movements: o Flexion and extension- transverse axis o Abduction and adduction- anteroposterior axis o Internal and external rotation- longitudinal axis • Greatest amount of motion and most unstable joint in body • Function: positions and directs humerus, elbow and hand in relation to trunk (radioulnar joint deal with palm and fingers) • Combines with scapulothoracic, acromioclavicular and sternoclavicular joints • Articular surfaces: o Humeral head- ½ sphere, faces medially o Glenoid fossa- very shallow, faces laterally o Only 25-30% contact between articular surfaces o Shallower and smaller surface compared to hip • Glenoid labrum o Glenoid labrum- fibrous structure around glenoid fossa (adheres to edges), central part avascular and edges not greatly vascularised à doesn’t heal well, labrum functions to; - Facilitate mobility - Increase glenoid concavity- increasing mobility - Provide attachment for joint capsule, ligaments and muscles • Joint capsule o Very thin and lax- doesn’t compromise ROM o Attaches to glenoid labrum o Reflected (folded) inferiorly onto medial shaft of humerus o Reinforced by; - Rotator cuff tendons- supraspinatus, infraspinatus, teres minor, subscapularis - Rotator cuff tendons- action: internal or external rotation, function: pull humerus head in against fossa - Glenohumeral and coracohumeral ligaments (capsular) • Synovial membrane o Lines -

Shotgun Mitogenomics Provides a Reference
Shotgun Mitogenomics Provides a Reference Phylogenetic Framework and Timescale for Living Xenarthrans Gillian Gibb, Fabien Condamine, Melanie Kuch, Jacob Enk, Nadia Moraes-Barros, Mariella Superina, Hendrik Poinar, Frédéric Delsuc To cite this version: Gillian Gibb, Fabien Condamine, Melanie Kuch, Jacob Enk, Nadia Moraes-Barros, et al.. Shotgun Mitogenomics Provides a Reference Phylogenetic Framework and Timescale for Living Xenarthrans. Molecular Biology and Evolution, Oxford University Press (OUP), 2016, 33 (3), pp.621 - 642. 10.1093/molbev/msv250. hal-01879331 HAL Id: hal-01879331 https://hal.archives-ouvertes.fr/hal-01879331 Submitted on 23 Sep 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial| 4.0 International License Shotgun Mitogenomics Provides a Reference Phylogenetic Framework and Timescale for Living Xenarthrans Gillian C. Gibb,1,2 Fabien L. Condamine,1,3,4 Melanie Kuch,5 Jacob Enk,5 Nadia Moraes-Barros,6,7 Mariella Superina,8 Hendrik N. Poinar,*,5 and Fred eric Delsuc*,1 -

The Conservation Game
From Penguins to Pandas - the conservation game Armadillo Fact Files © RZSS 2014 Armadillos There are 21 species of armadillo. They are divided into 8 groups: dwarf fairy giant hairy long nosed naked tail three banded yellow banded All armadillos have an outer covering of strong bony plates and horny skin. Most of the species can bring their legs in under this covering to protect themselves but it is only the three banded armadillos that can roll up into a tight ball. Most are active at night (nocturnal) and have poor eyesight but very good hearing and sense of smell. They all have large claws on their front feet to dig for insects and for burrowing. There is little known about some species of armadillo. At the time of writing (May 2014), the Royal Zoological Society of Scotland (RZSS) are studying four species - giant, southern naked tail, nine banded and yellow banded. Edinburgh Zoo, owned by RZSS holds 2 species, the larger hairy and the southern three banded. These animals are not on show but are sometimes seen in our animal presentations, such as ‘Animal Antics’. Armadillo in different languages Portuguese: tatu Spanish: armadillo (it means ‘little armoured one’ in Spanish) French: tatou Mandarin Chinese: 犰狳 (qiu yu) 2 Dwarf armadillo 1 species dwarf or pichi (Zaedyus pichiy) Distribution Argentina and Chile Diet insects, spiders and plants Breeding gestation 60 days litter size 1 - 2 Size Length: 26 - 33cm Tail length 10 - 14cm Weight 1 - 1.5kg Interesting facts comes out in daytime unlike most other species, it hibernates over -

Systematic Morphology of Fishes in the Early 21St Century
Copeia 103, No. 4, 2015, 858–873 When Tradition Meets Technology: Systematic Morphology of Fishes in the Early 21st Century Eric J. Hilton1, Nalani K. Schnell2, and Peter Konstantinidis1 Many of the primary groups of fishes currently recognized have been established through an iterative process of anatomical study and comparison of fishes that has spanned a time period approaching 500 years. In this paper we give a brief history of the systematic morphology of fishes, focusing on some of the individuals and their works from which we derive our own inspiration. We further discuss what is possible at this point in history in the anatomical study of fishes and speculate on the future of morphology used in the systematics of fishes. Beyond the collection of facts about the anatomy of fishes, morphology remains extremely relevant in the age of molecular data for at least three broad reasons: 1) new techniques for the preparation of specimens allow new data sources to be broadly compared; 2) past morphological analyses, as well as new ideas about interrelationships of fishes (based on both morphological and molecular data) provide rich sources of hypotheses to test with new morphological investigations; and 3) the use of morphological data is not limited to understanding phylogeny and evolution of fishes, but rather is of broad utility to understanding the general biology (including phenotypic adaptation, evolution, ecology, and conservation biology) of fishes. Although in some ways morphology struggles to compete with the lure of molecular data for systematic research, we see the anatomical study of fishes entering into a new and exciting phase of its history because of recent technological and methodological innovations.