A Model of Mass Extinction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lineages, Splits and Divergence Challenge Whether the Terms Anagenesis and Cladogenesis Are Necessary
Biological Journal of the Linnean Society, 2015, , – . With 2 figures. Lineages, splits and divergence challenge whether the terms anagenesis and cladogenesis are necessary FELIX VAUX*, STEVEN A. TREWICK and MARY MORGAN-RICHARDS Ecology Group, Institute of Agriculture and Environment, Massey University, Palmerston North, New Zealand Received 3 June 2015; revised 22 July 2015; accepted for publication 22 July 2015 Using the framework of evolutionary lineages to separate the process of evolution and classification of species, we observe that ‘anagenesis’ and ‘cladogenesis’ are unnecessary terms. The terms have changed significantly in meaning over time, and current usage is inconsistent and vague across many different disciplines. The most popular definition of cladogenesis is the splitting of evolutionary lineages (cessation of gene flow), whereas anagenesis is evolutionary change between splits. Cladogenesis (and lineage-splitting) is also regularly made synonymous with speciation. This definition is misleading as lineage-splitting is prolific during evolution and because palaeontological studies provide no direct estimate of gene flow. The terms also fail to incorporate speciation without being arbitrary or relative, and the focus upon lineage-splitting ignores the importance of divergence, hybridization, extinction and informative value (i.e. what is helpful to describe as a taxon) for species classification. We conclude and demonstrate that evolution and species diversity can be considered with greater clarity using simpler, more transparent terms than anagenesis and cladogenesis. Describing evolution and taxonomic classification can be straightforward, and there is no need to ‘make words mean so many different things’. © 2015 The Linnean Society of London, Biological Journal of the Linnean Society, 2015, 00, 000–000. -

A Framework for Post-Phylogenetic Systematics
A FRAMEWORK FOR POST-PHYLOGENETIC SYSTEMATICS Richard H. Zander Zetetic Publications, St. Louis Richard H. Zander Missouri Botanical Garden P.O. Box 299 St. Louis, MO 63166 [email protected] Zetetic Publications in St. Louis produces but does not sell this book. Any book dealer can obtain a copy for you through the usual channels. Resellers please contact CreateSpace Independent Publishing Platform of Amazon. ISBN-13: 978-1492220404 ISBN-10: 149222040X © Copyright 2013, all rights reserved. The image on the cover and title page is a stylized dendrogram of paraphyly (see Plate 1.1). This is, in macroevolutionary terms, an ancestral taxon of two (or more) species or of molecular strains of one taxon giving rise to a descendant taxon (unconnected comma) from one ancestral branch. The image on the back cover is a stylized dendrogram of two, genus-level speciational bursts or dis- silience. Here, the dissilient genus is the basic evolutionary unit (see Plate 13.1). This evolutionary model is evident in analysis of the moss Didymodon (Chapter 8) through superoptimization. A super- generative core species with a set of radiative, specialized descendant species in the stylized tree com- promises one genus. In this exemplary image; another genus of similar complexity is generated by the core supergenerative species of the first. TABLE OF CONTENTS Preface..................................................................................................................................................... 1 Acknowledgments.................................................................................................................................. -

September 2009
Coaster Brook Trout (Salvelinus fontinalis) 12-Month Finding Decision Document SEPTEMBER 2009 0 Coaster Brook Trout 12-Month Finding Decsion Document TABLE OF CONTENTS PURPOSE ............................................................................................................................................................... 1 BACKGROUND ........................................................................................................................................................... 1 DECISION STRUCTURE ........................................................................................................................................... 4 DECISION PROBLEM .................................................................................................................................................... 5 OBJECTIVES ................................................................................................................................................................. 7 ALTERNATIVES ............................................................................................................................................................. 7 CONSEQUENCES ........................................................................................................................................................ 10 TRADE-OFFS .............................................................................................................................................................. 13 DISTINCT POPULATION ANALYSIS ................................................................................................................. -

Speciation Free Download
SPECIATION FREE DOWNLOAD Jerry A. Coyne,H. Allen Orr | 480 pages | 09 Apr 2010 | Sinauer Associates Inc.,U.S. | 9780878930890 | English | Sunderland, United States speciation Categories : Speciation Ecology Evolutionary biology Sexual selection. Molecular Ecology. Hybrid zones are regions where diverged populations meet and interbreed. A Series of Books in Biology. John L. Buffalo grass seeds pass on the characteristics of the members in that region to offspring. Though vital to the concept of evolution, the Speciation "species" has been defined several different ways throughout history. Anolis lizardsa classroom activity for grades Speciation 30, Adaptation Natural selection Sexual selection Ecological speciation Assortative mating Haldane's rule. If an isolated population such as this survives its genetic upheavalsand subsequently expands into an unoccupied niche, or into a niche in which it has an advantage over its competitors, a new species, or subspecies, will Speciation come in being. The first and most commonly used sense refers to the "birth" of new species. Speciation possible Speciation of sympatric speciation is the apple maggot, an insect that lays its eggs inside the fruit of an apple, causing it to rot. Defining a species. See Speciation, and Darwin's dilemma. Pretty Fly The Hawaiian islands are home to Speciation of the most stunning Speciation of speciation. Ancestral wild cabbage. Plant Speciation. View Article. Princeton University Press. Fields and applications Applications of evolution Biosocial criminology Ecological genetics Evolutionary Speciation Evolutionary anthropology Evolutionary Speciation Evolutionary ecology Evolutionary economics Evolutionary epistemology Evolutionary ethics Evolutionary game theory Evolutionary linguistics Evolutionary medicine Evolutionary neuroscience Evolutionary physiology Evolutionary psychology Experimental evolution Phylogenetics Paleontology Selective breeding Speciation experiments Sociobiology Systematics Universal Darwinism. -

A Theory of Evolution Above the Species Level (Paleontology/Paleobiology/Speciation) STEVEN M
Proc. Nat. Acad. Sci. USA Vol. 72, No. 2, pp. 646-650, February 1975 A Theory of Evolution Above the Species Level (paleontology/paleobiology/speciation) STEVEN M. STANLEY Department of Earth and Planetary Sciences, The Johns Hopkins University, Baltimore, Maryland 21218 Communicated by Hanm P. Eugster, December 11, 1974 ABSTRACT Gradual evolutionary change by natural lished species are seen as occasionally becoming separated by selection operates so slowly within established species to form new species. Most isolates become that it cannot account for the major features of evolution. geographic barriers Evolutionary change tends to be concentrated within extinct, but occasionally one succeeds in blossoming into a speciation events. The direction of transpecific evolution is new species by evolving adaptations to its marginal environ- determined by the process of species selection, which is ment and diverging to a degree that interbreeding with the analogous to natural selection but acts upon species within original population is no longer possible. Mayr has empha- higher taxa rather than upon individuals within popula- tions. Species selection operates on variation provided sized the stability of the genotype of a typical established by the largely random process of speciation and favors species. Most species occupy heterogeneous environments species that speciate at high rates or survive for long within which selection pressures differ from place to place and periods and therefore tend to leave many daughter species. oppose each other through gene flow. Furthermore, biochemi- Rates of speciation can be estimated for living taxa by cal systems interact in complex ways. Most genes affect means of the equation for exponential increase, and are clearly higher for mammals than for bivalve mollusks. -

J. David Archibald
CURRICULUM VITAE J. DAVID ARCHIBALD AFFILIATION Department of Biology MAILING ADDRESS 4615 48th St San Diego State University San Diego, CA 92115-3206 5500 Campanile Dr. San Diego State University San Diego, CA 92182-4614 PHONE (619) 286-5521 FAX (619) 501-9587 E-MAIL [email protected] FACULTY PAGE http://www.bio.sdsu.edu/faculty/archibald.html EDUCATION B. Sc. in Geology, Kent State University, 1972, Magna cum Laude Ph. D. in Paleontology, University of California, Berkeley 1977 TEACHING EXPERIENCE AND ACADEMIC POSITIONS 1974-75: Teaching Assistant, Paleontology, University of California, Berkeley Courses assisted for: Introduction to Paleontology, undergraduate Morphology of the Vertebrate Skeleton, undergraduate Floras of the Past, undergraduate Phylogeny and Evolution, undergraduate 1977-79: J. Willard Gibbs Instructor (a postdoctoral fellowship), Dept. of Geology & Geophysics,Yale University Problems in Cretaceous-Paleogene Vertebrate Evolution, graduate seminar Evolution of Vertebrates to Man, undergraduate Paleobiology of Mammals, graduate Seminar in Primate Paleobiology (with D. Pilbeam) 1979-83: Assistant Professor (1979-1983) Associate Professor (promoted 1983, but did not accept promotion), Department of Biology, Yale University Vertebrate Anatomy, undergraduate Biologic Processes and Paleontologic Patterns, graduate seminar (with faculty from Biology, Geology, and Anthropology) Biology of Mammals, undergraduate Evolution, undergraduate (team taught) Paleobiology of Mammals, graduate Variability seminar (with faculty -

Medina and Goffinet
Review Better shoes alone don’t get you to your destination Reviewed by 1 RAFAEL MEDINA BERNARD GOFFINET Department of Ecology and Evolutionary Biology, 75 North Eagleville Road, University of Connecticut, Storrs, CT 06269-3043, U.S.A. 1e-mail: [email protected] Zander, R. H. 2013. A Framework for Post-Phylogenetic Systematics. Zetetic Publications, St. Louis, MO 209 p. [ISBN 978-1492220404]. Price $ 19.98 (softcover). ¤¤¤ A major challenge of reconstructing evolutionary rela- work is summarized (Chapter 16). The book includes a tionships is how to deal with distinct branching orders in glossary, which is essential given that the author uses trees inferred from different datasets, in particular terms likely to be unfamiliar to most readers (self-nesting morphological and molecular datasets. Topological in- ladder, caulistic taxa, extended paraphyly, etc.). congruences should be fully reconcilable since these The most important criticism expressed by Zander, characters are drawn from the same organisms and hence and somehow his principal motivation, is phylogenetic should reflect the outcome of the same underlying structuralism, the belief that evolutionary patterns are macroevolutionary process. Current widely applied best described in terms of the properties and rules of fundamental theoretical principles severely constrain scientific constructs, specifically Hennigian cladograms. the interpretation of phylogenetic trees and constitute The cladistic logic for phylogenetic reconstruction perhaps the primary, most significant obstacle to unifying in terms of dichotomous sister-group relationships is inferences in an integrative evolutionary reconstruction. A commonly accepted as the legitimate way to represent critical evaluation of the applicability of these principles to the diversification of lineages through time, yet may in reflect natural processes has become necessary, consider- fact, on theoretical grounds alone, fail to reflect ing that large data sets are generated with relative ease, and evolutionary processes. -

Block 4 SPECIATION and SPECIES EXTINCTION
BZYCT-137 GENETICS AND Indira Gandhi EVOLUTIONARY BIOLOGY National Open University School of Sciences Block 4 SPECIATION AND SPECIES EXTINCTION UNIT 14 Evolutionary Change, Species Concept and Speciation-I 127 UNIT 15 Evolutionary Change, Species Concept and Speciation-II 149 UNIT 16 Species Extinction 169 Course Design Committee Prof. M.S. Nathawat Dr. Ranjana Saxena Former Director, School of Sciences Associate Professor in Zoology IGNOU, Maidan Garhi, New Delhi-110068 Dyal Singh College, Lodhi Road, New Delhi-110003 Prof. S. S. Hasan (Retd.) Prof. Sarita Sachdeva School of Sciences, IGNOU Head, Department of Biotechnology Maidan Garhi, New Delhi-110068 Manav Rachna International Institute Prof. Jaswant Sokhi (Retd.) of Research and Studies, Faridabad, Haryana-121004 School of Sciences, IGNOU Prof. Neera Kapoor Maidan Garhi, New Delhi-110068 School of Sciences, IGNOU Prof. A.K. Bali Maidan Garhi, New Delhi-110068 Professor in Zoology Prof. Amrita Nigam Bhaskaracharya College of Applied Sciences School of Sciences, IGNOU Sector 2A, Dwarka, New Delhi-110075 Maidan Garhi, New Delhi-110068 Dr. S.K. Sagar Dr. Nisha Associate Professor in Zoology Consultant, School of Sciences Swami Shraddhanand College, Alipur Village IGNOU, New Delhi-110068 University of Delhi, Delhi- 110036 Dr. Anjali S. Nawani Dr. H.S. Pawar Consultant, School of Sciences Scientist D, NIMR IGNOU, New Delhi-110068 Sector 8, Dwarka, New Delhi-110077 Prof. Abhilasha Shourie Department of Biotechnology Manav Rachna International Institute of Research and Studies, Faridabad, Haryana-121004 Block Preparation Team Dr. H.S. Pawar School of Sciences Scientist D, NIMR, Sector 8, Dwarka Prof. Neera Kapoor (Units 14 to 16) New Delhi-110077 (Units 14 and 15) Dr. -
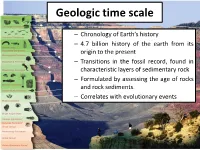
Geologic Time Scale
Geologic time scale – Chronology of Earth’s history – 4.7 billion history of the earth from its origin to the present – Transitions in the fossil record, found in characteristic layers of sedimentary rock – Formulated by assessing the age of rocks and rock sediments. – Correlates with evolutionary events Geologic time scale Based on geologic events the ancient period from earth’s history is formulated into eons-eras-periods-epochs. Each division in the geological calendar is clearly identified and described. Incidents pertaining to earth surface, plant and animal life are neatly recorded. The influence of geological and climatic changes on the life and the evolution of the living organism had been well analyzed. Earth is 4.7 billion (4,700 million) years old. Geologic time (4.7 billion/4,700 million) Divides geologic history into Divisions: four-level hierarchy of time intervals units • EONS Originally created using relative First and largest division of geologic time dates and more recently, Greatest expanse of time radioactive dating. Four eons The influence of geological and • Phanerozoic ("visible life") –most recent eon climatic changes on the life and • Proterozoic the evolution of the living • Archean organism • Hadean – the oldest eon • ERAS Second division of geologic time • PERIODS Third division of the geologic time. Named for either location or characteristics of the defining rock formations • EPOCHS Fourth division of geologic time Represents the subdivisions of a period . The geologic scale time The geologic PRECAMBRIAN SUPER EON 1. HADEAN EON (PRE-ARCHEAN EON) 4.6 to 3.8 billion years ago ~4.6 BYA -- Formation of Earth and Moon (as indicated by dating of meteorites and rocks from the Moon) ~4 BYA -- Likely origin of life -- indirect photosynthetic evidence of primordial life -- evidence of materials created by organic decay This is the "hidden" portion of geologic time as there is little evidence of this time remaining in Earth's rocks. -

The Pennsylvania State University the Graduate School Department
The Pennsylvania State University The Graduate School Department of Geosciences TAXIC AND PHYLOGENETIC APPROACHES TO UNDERSTANDING THE LATE ORDOVICIAN MASS EXTINCTION AND EARLY SILURIAN RECOVERY A Thesis in Geosciences by Andrew Z. Krug © 2006 Andrew Z. Krug Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy May 2006 The thesis of Andrew Z. Krug was reviewed and approved* by the following: Mark E. Patzkowsky Associate Professor of Geosciences Thesis Adviser Chair of Committee Timothy J. Bralower Professor of Geosciences Head of the Department of Geosciences Christopher H. House Assistant Professor of Geosciences Peter Wilf Assistant Professor of Geosciences Alan Walker Evan Pugh Professor of Anthropology and Biology *Signatures are on file in the Graduate School iii ABSTRACT The rules governing the accumulation and depletion of diversity vary at different geographic scales. Because of the spatially complex nature of the global ecosystem, understanding major macroevolutionary events requires an understanding of the processes that control diversity and turnover at a variety of geographic and temporal scales. This is of particular importance in the study of mass extinction events, which can eliminate established evolutionary lineages and ecologically dominant taxa, setting the stage for post-extinction radiation of previously obscure or minor lineages. If the macroevolutionary consequences of extinctions and recoveries are to be understood and predicted, the spatial and temporal variations in diversity and turnover must be quantified and the processes underlying these patterns dissected. Here, I analyze regional diversity and turnover patterns spanning the Late Ordovician mass extinction and Early Silurian recovery using a database of genus occurrences for inarticulate and articulate brachiopods, bivalves, anthozoans and trilobites. -

Package 'Paleotree'
Package ‘paleotree’ December 12, 2019 Type Package Version 3.3.25 Title Paleontological and Phylogenetic Analyses of Evolution Date 2019-12-12 Author David W. Bapst, Peter J. Wagner Depends R (>= 3.0.0), ape (>= 4.1) Imports phangorn (>= 2.0.0), stats, phytools (>= 0.6-00), jsonlite, graphics, grDevices, methods, png, RCurl, utils Suggests spelling, testthat Language en-US ByteCompile TRUE Encoding UTF-8 Maintainer David W. Bapst <[email protected]> BugReports https://github.com/dwbapst/paleotree/issues Description Provides tools for transforming, a posteriori time-scaling, and modifying phylogenies containing extinct (i.e. fossil) lineages. In particular, most users are interested in the functions timePaleoPhy, bin_timePaleoPhy, cal3TimePaleoPhy and bin_cal3TimePaleoPhy, which date cladograms of fossil taxa using stratigraphic data. This package also contains a large number of likelihood functions for estimating sampling and diversification rates from different types of data available from the fossil record (e.g. range data, occurrence data, etc). paleotree users can also simulate diversification and sampling in the fossil record using the function simFossilRecord, which is a detailed simulator for branching birth-death-sampling processes composed of discrete taxonomic units arranged in ancestor-descendant relationships. Users can use simFossilRecord to simulate diversification in incompletely sampled fossil records, under various models of morphological differentiation (i.e. the various patterns by which morphotaxa originate from one another), and with time-dependent, longevity-dependent and/or diversity-dependent rates of diversification, extinction and sampling. Additional functions allow users to translate simulated ancestor-descendant data from simFossilRecord into standard time-scaled phylogenies or unscaled cladograms that reflect the relationships among taxon units. -

Answers to Study and Review Questions
Answers to Study and Review Questions Answers are given below for calculations, problems, and short 4. It is an accident in the sense that other codes with the same answers; a reference to the relevant section(s) of the chapter is four letters could work equally well. It is frozen in the sense given for longer answers and definitions or explanations of that changes in it are selected against. See Section 3.8. technical terms; or a reference to further reading is given for 5. This is intended more as a discussion topic: for the idea see topics not explicitly discussed in the text. Section 3.9. 6. Because a form would have existed in time before the series Chapter 1 of fossils (of vertebrates from fish to mammals) that can be 1. See Section 1.1. strongly argued to be its ancestors. 2. Adaptation. 3. The popular concept had evolution as progressive, with Chapter 4 species ascending a one-dimensional line from lower 1. forms to higher. Evolution in Darwin’s theory is tree-like Number (as Proportion of Proportion of and branching, and no species is any “higher” than any density/m2) original cohort original cohort other a forms are adapted only to the environments they Age interval surviving to surviving to dying during live in. (days) day x day x interval 4. Darwin’s theory of natural selection and Mendel’s theory of heredity. 0–250 100 100 0.9989 251–500 0.11 0.11 0.273 Chapter 2 501–750 0.08 0.08 0.75 1.