Benzodiazepine Evidence Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Once-Daily Skeletal Muscle Relaxant in SA
NEWS Once-daily skeletal muscle relaxant in SA Myprocam (cyclobenzaprine with spine-related disorders and other 8. What are the classes of muscle a protective coating, and a polymer extended release [ER] sports injuries are excellent candidates relaxants? membrane that controls the rate of hydrochloride) is the most widely for Myprocam. Skeletal muscle relaxants (SMRs) are drug release. prescribed skeletal muscle a group of structurally unrelated drugs, The formulation of the drug relaxant in the US, is now available 4. What are the common as shown in Figure 1. These are divided ensures early systemic exposure in SA. Myprocam’s safety and efficacy indications for which Myprocam into two categories: to cyclobenzaprine, with a plasma has been shown in more than 20 clinical is used, and what are the common • Antispasticity agents: Used to concentration at four hours that trials. It was recently launched by causes of these ailments? treat muscle spasticity caused by is similar to that observed with Adcock Ingram nationally, making SA Low back pain, neck pain, sports traumatic neurologic injury, multiple cyclobenzaprine IR. the second country (only to the US) to injuries, muscle sprains and strains. sclerosis, and other conditions. However, in contrast to the bring the product to market. Common causes of these conditions • Antispasmodic agents: Used fluctuating peaks and troughs in Myprocam, indicated for the relief of include sports injuries, car accidents, to treat muscular pain or spasm plasma cyclobenzaprine concentration muscle spasm associated with acute work-related injuries, and everyday associated with acute, nonspecific after administration of the IR and painful musculoskeletal conditions, activities, which account for acute musculoskeletal conditions. -

22 Psychiatric Medications for Monitoring in Primary Care
22 Psychiatric Medications for Monitoring in Primary Care Medication Warnings, Precautions, and Adverse Events Comments Class: SSRI Fluvoxamine Boxed Warnings: Suicidality Used much less than SSRIs in the group of eight Indications: Warnings and Precautions: Similar to other SSRIs medications for prescribing, probably because it has no Adult: OCD Adverse Events: Similar to other SSRIs FDA indication for MDD or any anxiety disorder. Still Child/Adolescent: OCD (10-17 years) somewhat popular as a medication for OCD. Uses: Anxiety, OCD Monitoring: Same as other SSRIs Citalopram Boxed Warning: Suicidality. Escitalopram, one of the SSRIs in the group of Indications: Warnings and Precautions: Similar to other SSRIs medications for prescribing, is an active metabolite of Adult: MDD Adverse Events: Similar to other SSRIs citalopram. Escitalopram reportedly has fewer AEs and Child/Adolescent: None less interaction with hepatic metabolic enzymes than Uses: Anxiety, MDD, OCD citalopram but is otherwise essentially identical. Citalopram offers no advantage other than price, as Monitoring: Same as other SSRIs escitalopram is branded until 2012. Paroxetine Boxed Warnings: Suicidality. Paroxetine used much less than the SSRIs for Indications: Warnings and Precautions: Similar to other SSRIs prescribing, probably because of its nonlinear kinetics. Adult: MDD, OCD, Panic Disorder, Generalized Anxiety Adverse Events: Similar to other SSRIs A study of children and adolescents showed doubling Disorder, Social Anxiety Disorder, Posttraumatic Stress Disorder the dose of paroxetine from 10 mg/day to 20 mg/day Child/Adolescent: None resulted in a 7-fold increase in blood levels (Findling et Uses: Anxiety, MDD, OCD al, 1999). Thus, once metabolic enzymes are saturated, paroxetine levels can increase dramatically with dose Monitoring: Same as other SSRIs increases and decrease dramatically with dose decreases, sometimes leading to adverse events. -

Benzodiazepine Anti-Anxiety Agents: Prevalence and Correlates of Use in a Southern Community
Benzodiazepine Anti-anxiety Agents: Prevalence and Correlates of Use in a Southern Community rn- rn Marvin Swartz, MD, Richard Landerman, PhD, Linda K George, PhD, Mary Lou Melville, MD, Dan Blazer, MD, PhD, and Karen Smith, PhD Introduction alence and patterns of benzodiazepine antianxiolytic drug use in the Piedmont re- Benzodiazepine anti-anxiety agents gion of North Carolina during 1982-83, uti- are the most widely prescribed psycho- lizing logistic regression analysis, which al- therapeutic drugs in the United States to- lows prediction of benzodiazepine use day.' First introduced in 1960, these drugs while introducing controls for potential rapidly achieved a lead position in the pre- confounding and mediating variables. scription drug market,2 stimulating public and professional debate over appropriate Method psychotropic drug use.3 Recent evidence, however, suggests that the prevalence and The present paper reports results patterns of psychotropic use, especially from Wave 1 of the Piedmont Health Sur- those of benzodiazepine anxiolytics, may vey, one site of the five-site National In- be changing and resulting in decreased stitute of Mental Health Epidemiologic use.4,5 The first detailed population survey Catchment Area program (NIMH- of psychotropic drug use, the National ECA).'1 The sampling frame for the Pied- Household Sample in 1970-71,6-9 found mont Health Survey (PHS) was a five- that 22 percent of American adults had county area in north central North used prescription psychotropic medica- Carolina, consisting of one urban county tion during the year 1969-70, with higher and four contiguous rural counties. The use among women and the elderly. -

(Orion) 5 Mg Tablets Buspirone (Orion) 10 Mg Tablets
NEW ZEALAND DATA SHEET 1. PRODUCT NAME Buspirone (Orion) 5 mg tablets Buspirone (Orion) 10 mg tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 5 mg buspirone hydrochloride. Each tablet contains 10 mg buspirone hydrochloride. Excipient with known effect: 5 mg tablet: Each tablet contains 59.5 mg lactose (as monohydrate) 10 mg tablet: Each tablet contains 118.9 mg lactose (as monohydrate). For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Tablet. 5 mg tablet: White or almost white, oval tablets debossed with ‘ORN 30’ on one side and a score on the other side. The tablet can be divided into equal doses. 10 mg tablet: White or almost white, oval tablets debossed with ‘ORN 31’ on one side and a score on the other side. The tablet can be divided into equal doses. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Buspirone hydrochloride is indicated for the management of anxiety with or without accompanying depression in adults. Buspirone hydrochloride is indicated for the management of anxiety disorders or the short- term relief of symptoms of anxiety with or without accompanying depression. 4.2 Posology and method of administration The usual starting dose is 5 mg given three times daily. This may be titrated according to the needs of the patient and the daily dose increased by 5 mg increments every two or three days depending upon the therapeutic response to a maximum daily dose of 60 mg. After dosage titration the usual daily dose will be 20 to 30 mg per day in divided doses. -

Calcium Channel Blockers in Mental Health and Neuro Sciences
Article NIMHANS Journal Editorial : Calcium Channel Blockers in Mental Health and Neuro Sciences Volume: 15 Issue: 01 January 1997 Page: 3-5 S M Channabasavanna, - Vice-Chancellor, NIMHANS, (Deemed University), Bangalore Calcium is a familiar ion; first year medical students learn of its physiological importance in a host of situations, such as in nutrition, in enzymatic reactions, in muscle contraction, and in the structure of bone. Calcium has long been known to also play an important role in the physiology of the nervous system, for example, it is well recognised that calcium is a co-factor in several enzymatic processes in the central nervous system (CNS), that release of neurotransmitters from synaptic vesicles is calcium-dependent, that the stability of the neuronal membrane is partly regulated by calcium, that calcium is involved in neurotoxic processes etc. [1]. Several cognitive functions have been associated with calcium mechanisms. For example, the involvement of calcium is learning and memory processes is widely documented [2], [3]; most of the work to-date concerns calcium influx through receptors for excitatory amino acids [4]; compounds that block inonotropic glutamate receptors and inhibit calcium inflow, such as MK 801, are reported to impair memory [5], [6]. Voltage-dependent calcium channels, of which the best characterized are those that contain dihydropyridine binding sites, form a second mechanism controlling calcium inflow into the cells [7]. In recent years, these calcium channels in the neuron have assumed substantial importance; treatments affecting these channels have been shown to affect a number of functions related to the CNS. For example, repeated electroconvulsive shocks upregulate voltage dependent calcium channels, which effect appears to increase sensitivity to pain, enhance responsiveness to dopaminergic agonists etc [8], [9], [10]. -
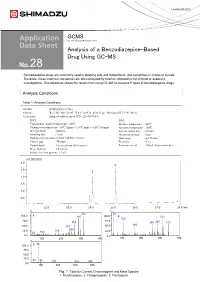
Analysis of a Benzodiazepine-Based Drug Using GC-MS 28
LAAN-E-MS-E028 GCMS Gas Chromatograph Mass Spectrometer Analysis of a Benzodiazepine-Based Drug Using GC-MS 28 Benzodiazepine drugs are commonly used in sleeping aids and tranquilizers, and sometimes in crimes or suicide. Therefore, these chemical substances are often analyzed by forensic laboratories for criminal or academic investigations. This datasheet shows the results from using GC-MS to measure 9 types of benzodiazepine drugs. Analysis Conditions Table 1: Analysis Conditions GC-MS :GCMS-QP2010 Ultra Column : Rxi®-5Sil MS (30 mL. X 0.25 mmI.D., df=0.25 µm, Shimadzu GLC P/N:13623) Glass insert :Silanized splitless insert (P/N: 221-48876-03) [GC] [MS] Vaporization chamber temperature : 260℃ Interface temperature : 280℃ Column oven temperature : 60℃ (2min) -> (10℃/min) -> 320℃ (10min) Ion source temperature : 200℃ Injection mode : Splitless Solvent elution time : 2.0 min Sampling time : 1 min Measurement mode : Scan High pressure injection method: 250 kPa (1.5 min) Mass range : m/z 35-600 Carrier gas : Helium Event time : 0.3 sec Control mode :Linear velocity (45.6 cm/sec) Emission current : 150 µA (high sensitivity) Purge flow rate :3.0 ml/min Sample injection quantity :1.0 µL (x1,000,000) 3.0 3 2.5 2 2.0 1.5 1.0 1 0.5 22.0 23.0 24.0 25.0 26.0 27.0 28.0 min % % 312 55 100.0 1 285 100.0 2 313 109 75.0 75.0 266 259287 342 166 50.0 238 50.0 248 25.0 183 25.0 63 109 0.0 0.0 100 200 300 400 100 200 300 400 % 86 100.0 3 75.0 50.0 25.0 58 99 183 315 387 0.0 100 200 300 400 Fig. -

Recommended Methods for the Identification and Analysis of Fentanyl and Its Analogues in Biological Specimens
Recommended methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES Laboratory and Scientific Section UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Recommended Methods for the Identification and Analysis of Fentanyl and its Analogues in Biological Specimens MANUAL FOR USE BY NATIONAL DRUG ANALYSIS LABORATORIES UNITED NATIONS Vienna, 2017 Note Operating and experimental conditions are reproduced from the original reference materials, including unpublished methods, validated and used in selected national laboratories as per the list of references. A number of alternative conditions and substitution of named commercial products may provide comparable results in many cases. However, any modification has to be validated before it is integrated into laboratory routines. ST/NAR/53 Original language: English © United Nations, November 2017. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Mention of names of firms and commercial products does not imply the endorse- ment of the United Nations. This publication has not been formally edited. Publishing production: English, Publishing and Library Section, United Nations Office at Vienna. Acknowledgements The Laboratory and Scientific Section of the UNODC (LSS, headed by Dr. Justice Tettey) wishes to express its appreciation and thanks to Dr. Barry Logan, Center for Forensic Science Research and Education, at the Fredric Rieders Family Founda- tion and NMS Labs, United States; Amanda L.A. -

Drugs Used to Treat Joint and Muscle Disease
PHARMACOLOGY Drugs used to treat joint and Learning objectives muscle disease After reading this article, you should be able to: David G Lambert C list the main joint and muscle diseases and their treatments C describe the treatment options for arthritic disease, myasthenia gravis and muscle spasticity Abstract Joint disease: Arthritis can be simply broken into osteoarthritis and rheumatoid arthritis (RA). Osteoarthritis is treated with symptomatic pain relief and surgery. RA is a chronic autoimmune disease that causes 1. Osteoarthritis: this is the most common joint disease and is inflammation of joints (leading to their destruction), tissues around joints often described as a disease of wear and tear. This disease affects and other organ systems. Treatment (for pain) of RA in the first instance is around 8.5 million people in the UK alone, is more common in with non-steroidal anti-inflammatory drugs, with second-line treatment women and those who are overweight and affects a range of using disease-modifying antirheumatic drugs (DMARDs). DMARDs are a joints (knees, hips, hands and neck). There are important disparate group and include methotrexate, D-penicillamine, sulphasala- anaesthetic consequences relating to a reduced range of move- zine, gold salts, antimalarial drugs and immunosuppressant drugs. The ment when the neck is affected. Typical degenerative changes newer class of ‘biological’ DMARDs includes etanercept (tumour necrosis include a thinning of joint cartilage, growth of osteophytes and factor a (TNF-a) receptoreimmunoglobulin G chimera), infliximab (mono- increased synovial fluid production. Typical presenting symp- clonal anti-TNF-a antibody), anakinra (interleukin 1 receptor antagonist) toms are pain, joint stiffness, swelling and a reduced range of and rituximab (an anti-CD20 antibody that depletes B cells). -

124.210 Schedule IV — Substances Included. 1
1 CONTROLLED SUBSTANCES, §124.210 124.210 Schedule IV — substances included. 1. Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. 2. Narcotic drugs. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below: a. Not more than one milligram of difenoxin and not less than twenty-five micrograms of atropine sulfate per dosage unit. b. Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2- propionoxybutane). c. 2-[(dimethylamino)methyl]-1-(3-methoxyphenyl)cyclohexanol, its salts, optical and geometric isomers and salts of these isomers (including tramadol). 3. Depressants. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances, including its salts, isomers, and salts of isomers whenever the existence of such salts, isomers, and salts of isomers is possible within the specific chemical designation: a. Alprazolam. b. Barbital. c. Bromazepam. d. Camazepam. e. Carisoprodol. f. Chloral betaine. g. Chloral hydrate. h. Chlordiazepoxide. i. Clobazam. j. Clonazepam. k. Clorazepate. l. Clotiazepam. m. Cloxazolam. n. Delorazepam. o. Diazepam. p. Dichloralphenazone. q. Estazolam. r. Ethchlorvynol. s. Ethinamate. t. Ethyl Loflazepate. u. Fludiazepam. v. Flunitrazepam. w. Flurazepam. x. Halazepam. y. Haloxazolam. z. Ketazolam. aa. Loprazolam. ab. Lorazepam. ac. Lormetazepam. ad. Mebutamate. ae. Medazepam. af. Meprobamate. ag. Methohexital. ah. Methylphenobarbital (mephobarbital). -

S1 Table. List of Medications Analyzed in Present Study Drug
S1 Table. List of medications analyzed in present study Drug class Drugs Propofol, ketamine, etomidate, Barbiturate (1) (thiopental) Benzodiazepines (28) (midazolam, lorazepam, clonazepam, diazepam, chlordiazepoxide, oxazepam, potassium Sedatives clorazepate, bromazepam, clobazam, alprazolam, pinazepam, (32 drugs) nordazepam, fludiazepam, ethyl loflazepate, etizolam, clotiazepam, tofisopam, flurazepam, flunitrazepam, estazolam, triazolam, lormetazepam, temazepam, brotizolam, quazepam, loprazolam, zopiclone, zolpidem) Fentanyl, alfentanil, sufentanil, remifentanil, morphine, Opioid analgesics hydromorphone, nicomorphine, oxycodone, tramadol, (10 drugs) pethidine Acetaminophen, Non-steroidal anti-inflammatory drugs (36) (celecoxib, polmacoxib, etoricoxib, nimesulide, aceclofenac, acemetacin, amfenac, cinnoxicam, dexibuprofen, diclofenac, emorfazone, Non-opioid analgesics etodolac, fenoprofen, flufenamic acid, flurbiprofen, ibuprofen, (44 drugs) ketoprofen, ketorolac, lornoxicam, loxoprofen, mefenamiate, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, pranoprofen, proglumetacin, sulindac, talniflumate, tenoxicam, tiaprofenic acid, zaltoprofen, morniflumate, pelubiprofen, indomethacin), Anticonvulsants (7) (gabapentin, pregabalin, lamotrigine, levetiracetam, carbamazepine, valproic acid, lacosamide) Vecuronium, rocuronium bromide, cisatracurium, atracurium, Neuromuscular hexafluronium, pipecuronium bromide, doxacurium chloride, blocking agents fazadinium bromide, mivacurium chloride, (12 drugs) pancuronium, gallamine, succinylcholine -

Drug Screening: Actual Status, Pitfalls and Suggestions for Improvement Drogenscreening: Gegenwa¨ Rtiger Stand, Fehlermo¨ Glichkeiten Und Verbesserungsvorschla¨Ge
J Lab Med 2004;28(4):317–325 ᮊ 2004 by Walter de Gruyter • Berlin • New York 2004/03304 Drug Monitoring und Toxikologie Redaktion: V. W. Armstrong Drug screening: Actual status, pitfalls and suggestions for improvement Drogenscreening: Gegenwa¨ rtiger Stand, Fehlermo¨ glichkeiten und Verbesserungsvorschla¨ge Wolf R. Ku¨ lpmann* pretierbar. In Wirklichkeit mu¨ ssen viele Aspekte beru¨ ck- sichtigt werden, um einen aussagekra¨ ftigen Befund zu Klinische Chemie, Medizinische Hochschule Hannover, erhalten, z.B. Pra¨ analytik, Spezifita¨ t und Empfindlichkeit Hannover, Germany des Verfahrens oder Qualita¨ tssicherung. Obwohl die Abstract mechanisierten Meßverfahren naturgema¨ ß quantitative Ergebnisse liefern, wird aufgezeigt, daß es sich in der Immunoassays for drug screening are often regarded as Regel empfiehlt, qualitative Befunde zu erstellen. Die procedures which are easily performed and interpreted. Verfahren erlauben aber im Gegensatz zu den auch fu¨r But in fact, many aspects have to be considered to diese Zwecke verwendeten Teststreifen die Entschei- obtain a meaningful result. Among these are sampling, dungsgrenze (cut-off) der jeweiligen Fragestellung anzu- specificity and sensitivity of assays, and quality assess- passen, z.B. bei akuter Intoxikation, chronischem Abusus ment. Although the mechanised procedures yield quan- oder U¨ berwachung des Drogenentzugs. titative results, there are good reasons to generally report Ein schwerwiegender Nachteil von Immunoassays zum qualitative findings. The mechanised procedures allow Nachweis von Amphetamin und a¨ hnlichen Substanzen, the adjustment of the cut-off concentration to the clinical Barbituraten, Benzodiazepinen und Opiaten besteht dar- setting, e.g. in the case of acute intoxication, chronic in, daß eine starke Kreuzreaktivita¨ t des Antiko¨ rpers abuse or drug withdrawal, an important advantage as gegenu¨ ber pharmakologisch wenig wirksamen Verbin- compared to test strips. -

Medications in Long-Term Care: When Less Is More
Medications in Long-Term Care: When Less is More a, b,c Thomas W. Meeks, MD *, John W. Culberson, MD , d,e Monica S. Horton, MD, MSc KEYWORDS Long-term care Inappropriate prescribing Polypharmacy Psychotropics Opiates Sedatives HISTORY OF MEDICATION REDUCTION IN LONG-TERM CARE The Nursing Home Reform Act (OBRA-87) enacted in 1987 called for sweeping changes in the standards of care in nursing homes in accordance with new, more demanding federal regulations. For example, OBRA-87 called for a new approach to the use of antipsychotics in persons with dementia. Because antipsychotics were regarded as frequently inappropriate chemical restraints in long-term care (LTC), OBRA-87 mandated dose reductions in antipsychotics in an effort to discontinue them whenever possible. OBRA-87 proposed that a safer, more supportive environ- ment in LTC settings would facilitate such reductions in antipsychotic doses.1 Overall rates of potentially inappropriate prescribing in older adults have ranged from 12% to 40%, depending on the setting, criteria, and population sampled.2 Devel- oping from burgeoning concerns for polypharmacy and potential iatrogenic toxicity of medication in older adults, expert consensus lists of potentially inappropriate pharma- cotherapy in the elderly (PIPE) began to emerge. In general, drugs with activity in the central nervous system (CNS) were commonly placed on these PIPE lists, and hence our focus on such medications in this review. This work was supported by the following Geriatric Academic Career Awards: (1) K01 HP00047-02 (Dr. Meeks) (2) K01 HP00080-02 (Dr. Culberson) (3) K01 HP00114-02 (Dr. Horton). The authors have no conflicts of interest to disclose.