Pacific Northwest Potato Production - REVISION
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pest Management Strategic Plan for Organic Potato Production in the West
Pest Management Strategic Plan for Organic Potato Production in the West Summary of workshops held on February 16, 2006 Buhl, Idaho and January 9, 2008 Portland, Oregon Issue Date December 19, 2008 Lead Authors: Jennifer Miller, Ronda Hirnyck, Lisa Downey-Blecker Editor: Diane Clarke This project was sponsored by the Western Integrated Pest Management Center, which is funded by the United States Department of Agriculture, Cooperative State Research, Education, and Extension Service. Additional funding was provided by the Organic Farming Research Foundation and the Bullitt Foundation. Table of Contents Work Group .........................................................................................................................3 Summary of the Most Critical Needs in Organic Potato Production in the West ................5 Introduction ..........................................................................................................................6 Production Overview .........................................................................................................11 California ...............................................................................................................11 Colorado .................................................................................................................12 Columbia Basin ......................................................................................................12 Idaho ......................................................................................................................13 -

PCN Guidelines, and Potato Cyst Nematodes (Globodera Rostochiensis Or Globodera Pallida) Were Not Detected.”
Canada and United States Guidelines on Surveillance and Phytosanitary Actions for the Potato Cyst Nematodes Globodera rostochiensis and Globodera pallida 7 May 2014 Table of Contents 1. Introduction ...........................................................................................................................................................3 2. Rationale for phytosanitary actions ........................................................................................................................3 3. Soil sampling and laboratory analysis procedures .................................................................................................4 4. Phytosanitary measures ........................................................................................................................................4 5. Regulated articles .................................................................................................................................................5 6. National PCN detection survey..............................................................................................................................6 7. Pest-free places of production or pest-free production sites within regulated areas ...............................................6 8. Phytosanitary certification of seed potatoes ..........................................................................................................7 9. Releasing land from regulatory control ..................................................................................................................8 -
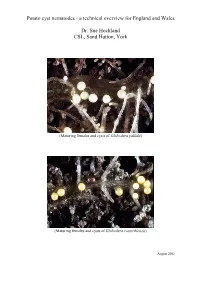
Potato Cyst Nematodes - a Technical Overview for England and Wales
Potato cyst nematodes - a technical overview for England and Wales Dr. Sue Hockland CSL, Sand Hutton, York (Maturing females and cysts of Globodera pallida) (Maturing females and cysts of Globodera rostochiensis) August 2002 Contents Page • Executive Summary 1 • Introduction 2 • PCN: species and diagnosis 2 • Biology of PCN species 3 • Detection 4 • Host plants of PCN 4 • Symptoms 5 • Damage 5 • Pathotypes and Host Plant Resistance 5 • Distribution and spread in England and Wales 6 • Distribution in Europe and elsewhere 9 • Statutory Management of PCN 11 • Management of PCN in ware potatoes 13 • Chemical methods 14 • Non-chemical methods 14 • Conclusion 16 • Acknowledgements 17 Executive Summary The pests Potato cyst nematode (PCN) is the name commonly given to two species of cyst nematode that attack potato, namely Globodera pallida (Stone) Behrens and G. rostochiensis (Wollenweber) Behrens. They are two of the most important pests of potato in England and Wales, feeding on potato roots, causing losses of yield and costs that vary and are difficult to estimate. Published papers usually quote losses of about 9% of annual yield, estimated at about £43 million for the UK, based on the mean value of the crop from 1990-1995. Adaptations to a plant-parasitic life In both species the female forms a hard covering around her eggs when she dies, creating a ‘cyst’ which protects the eggs and developing juveniles from desiccation, predation and chemical control. Only a proportion of the eggs hatch from the cyst each year, and in G. pallida this occurs at a slower rate and with a later annual peak of hatching than G. -

Pale Cyst Nematode Globodera Pallida
Michigan State University’s invasive species factsheets Pale cyst nematode Globodera pallida The pale cyst nematode is a serious pest of potatoes around the world and is a target of strict regulatory actions in the United States. An introduction to Michigan may adversely affect production and marketing of potatoes and other solanaceous crops. Michigan risk maps for exotic plant pests. Other common names potato cyst nematode, white potato cyst nematode, pale potato cyst nematode Systematic position Nematoda > Tylenchida > Heteroderidae > Globodera pallida (Stone) Behrens Global distribution Worldwide distribution in potato-producing regions. Mature females (white) and a cyst (brown) of potato cyst nematode attached to the roots. (Photo: B. Hammeraas, Bioforsk - Norwegian Institute for Agricultural Africa: Algeria, Tunisia; Asia: India, Pakistan, and Environmental Research, Bugwood.org) Turkey; Europe: Austria, Belgium, Bulgaria, Croatia, Czech Republic, Cyprus, Faroe Islands, Finland, pale cyst nematode move to, penetrate and feed on host France, Germany, Greece, Hungary, Iceland, Ireland, roots. After mating, fertilized eggs develop inside females Italy, Luxembourg, Malta, Netherlands, Norway, Poland, that are attached to roots. When females die their skin Portugal, Romania, Spain, Sweden, Switzerland, United hardens and becomes a protective brown cover (cyst) Kingdom; Latin America: Argentina, Bolivia, Chile, around the eggs. Each cyst contains hundreds of eggs, and Colombia, Ecuador, Panama, Peru, Venezuela; Oceania: it can remain viable for many years in the absence of host New Zealand; North America: Idaho, Canada (a small plants. One generation normally occurs during one growing area of Newfoundland). season. Quarantine status Identification In 2006, the pale cyst nematode was detected at At flowering or later stages of a host plant, mature a potato processing facility in Idaho. -

PLANT-PARASITIC NEMATODES in FIELD PEA and POTATO and THEIR EFFECT on PLANT GROWTH and YIELD a Thesis Submitted to the Graduate
PLANT-PARASITIC NEMATODES IN FIELD PEA AND POTATO AND THEIR EFFECT ON PLANT GROWTH AND YIELD A Thesis Submitted to the Graduate Faculty of the North Dakota State University of Agriculture and Applied Science By Arjun Upadhaya In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Major Department: Plant Pathology June 2018 Fargo, North Dakota North Dakota State University Graduate School Title PLANT-PARASITIC NEMATODES IN FIELD PEA AND POTATO AND THEIR EFFECT ON PLANT GROWTH AND YIELD By Arjun Upadhaya The Supervisory Committee certifies that this disquisition complies with North Dakota State University’s regulations and meets the accepted standards for the degree of MASTER OF SCIENCE SUPERVISORY COMMITTEE: Dr. Guiping Yan Chair Dr. Julie Pasche Dr. Andrew Robinson Approved: June 11, 2018 Dr. Jack Rasmussen Date Department Chair ABSTRACT In this study, surveys were conducted in pea and potato fields in North Dakota and Central Minnesota to investigate the incidence and abundance of plant-parasitic nematodes in these fields. Moreover, the effect of the pin nematode, Paratylenchus nanus, on plant growth and yield of six field pea cultivars was determined under greenhouse conditions. Similarly, the influence of lesion nematode, Pratylenchus penetrans, and wilt fungi, Fusarium oxysporum alone and together on growth and yield of potato cultivar ‘Red Norland’, was evaluated in microplots under field conditions. The results indicate Paratylenchus spp. and Pratylenchus spp. are the most frequent nematodes, respectively, in pea and potato fields. Pin nematodes reproduced on field pea cultivars and caused up to 37% reduction in plant height and 40% reduction in yield. -

Integrated Pest Management Strategic Plan for Potatoes in Oregon, Washington and Idaho
OREGON STATE UNIVERSITY EXTENSION SERVICE Integrated Pest Management Strategic Plan for Potatoes in Oregon, Washington and Idaho Summary of a workshop held on Feb. 22, 2019 Portland, Oregon Katie Murray, Paul Jepson, Isaac Sandlin, Oregon State University; Andy Jensen, NW Potato Research Consortium EM 9275 February 2020 Table of contents Process for this integrated pest management Potato pest management timing by crop stage 20 strategic plan 3 Major potato pest descriptions 21 Work group members 4 Potato pest management activities by crop stage 34 Review of 2006 summary of the most critical needs Planting to pre-emergence (March–May with in Pacific Northwest potato pest management 5 regional differences) 38 2019 top-priority critical needs 8 Emergence to row closure (April–early/late June) 41 Potato production overview 9 Row closure to harvest (June- September/October) 45 Integrated pest management overview in potato Harvest to postharvest 51 production 11 Invasive and emerging pests 53 MRL/export issues 13 References 54 Pollinator protection in potato production 14 Appendices 55 IPM critical needs 15 Using PAMS Terminology 85 List of major potato pests 17 Pesticide Risk Classification 86 This project was sponsored with funding from the Extension Implementation program of the National Institute of Food and Agriculture, United States Department of Agriculture (award No. 2017-70006-27154), with co-funding from the potato commissions of Idaho, Oregon, and Washington through the NW Potato Research Consortium. The IPMSP format is research in progress. Contact: Katie Murray Integrated Plant Protection Center 2040 Cordley Hall, Oregon State University Corvallis, OR 97331-2915 [email protected] Katie Murray, statewide IPM coordinator, associate professor of practice, Oregon IPM Center; Paul Jepson, professor (retired), Oregon IPM Center; Isaac Sandlin, faculty research assistant, Oregon IPM Center, all of Oregon State University; and Andy Jensen, manager, NW Potato Research Consortium. -

PNACJ008.Pdf
ptJ - Ac-:s-oog. '$-14143;1' mM1drtdffiii,tiifflj!:tl{ftj1f!f.ji{§,,{9,'tft'B4",]·'6M" No.19• Potato Colin J. Jeffries in collaboration with the Scottish Agricultural Science Agency _;~S~_ " -- J J~ IPGRI IS a centre ofthe Consultative Group on InternatIOnal Agricultural Research (CGIARl 2 FAO/lPGRI Technical Guidelines for the Safe Movement of Germplasm [Pl"e'\J~olUsiy Pub~~shed lrechnk:::aJi GlUio1re~~nes 1101" the Saffe Movement of Ger(m[lJ~Z!sm These guidelines describe technical procedures that minimize the risk ofpestintroductions with movement of germplasm for research, crop improvement, plant breeding, exploration or conservation. The recom mendations in these guidelines are intended for germplasm for research, conservation and basic plant breeding programmes. Recommendations for com mercial consignments are not the objective of these guidelines. Cocoa 1989 Edible Aroids 1989 Musa (1 st edition) 1989 Sweet Potato 1989 Yam 1989 Legumes 1990 Cassava 1991 Citrus 1991 Grapevine 1991 Vanilla 1991 Coconut 1993 Sugarcane 1993 Small fruits (Fragaria, Ribes, Rubus, Vaccinium) 1994 Small Grain Temperate Cereals 1995 Musa spp. (2nd edition) 1996 Stone Fruits 1996 Eucalyptus spp. 1996 Allium spp. 1997 No. 19. Potato 3 CONTENTS Introduction .5 Potato latent virus 51 Potato leafroll virus .52 Contributors 7 Potato mop-top virus 54 Potato rough dwarf virus 56 General Recommendations 14 Potato virus A .58 Potato virus M .59 Technical Recommendations 16 Potato virus P 61 Exporting country 16 Potato virus S 62 Importing country 18 Potato virus -

Potato Cyst Nematodes Factsheet
Biosecurity SA – Plant Health Exotic Plant Pest Hotline: 1800 084 881 (available 24 hours) Email [email protected] June 2017 Potato cyst nematodes Globodera pallida and Globodera rostochiensis WHAT IS IT? Pale potato cyst nematode (Globodera pallida) is an exotic plant pest not present in Australia Golden potato cyst nematode (Globodera rostochiensis) is present in some areas of Victoria but is not in South Australia. These nematodes are a serious threat to Australia’s potato industry. Potato cyst nematodes (PCN) are soil-borne microscopic worms which feed on roots of potato plants. Root development and tuber yield is reduced and plant growth is stunted. The biology and symptoms caused by both species Crop damage caused by PCN. are similar. Photo courtesy© Syngenta 2013 The occurrence of PCN is related to the presence of a host and not to soil type or soil temperature. The preferred host of PCN is potato. PCN can infest plants such as tomato, eggplant and some solanaceous weeds. PCN can survive as cysts in the soil for many years in the absence of host plants. PCN is subject to stringent quarantine and regulatory procedures wherever it occurs. WHAT TO LOOK FOR? The symptoms of attack by Globodera species are not specific. Symptoms may appear similar to water or nutrient deficiencies or wilt diseases. Infested potato plants have a reduced root system which is abnormally branched and brownish in colour. Growth is stunted, leaves yellow early or turn a dull colour, flowering is delayed and plants may wilt. At or after flowering very tiny white, yellow or brown cysts about the size of a pin head (0.5 mm) might be seen on the outside of roots HOW DOES IT SURVIVE? PCN are small worms less than 1 mm in size. -

Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: a Review
plants Review Plant Viruses Infecting Solanaceae Family Members in the Cultivated and Wild Environments: A Review Richard Hanˇcinský 1, Daniel Mihálik 1,2,3, Michaela Mrkvová 1, Thierry Candresse 4 and Miroslav Glasa 1,5,* 1 Faculty of Natural Sciences, University of Ss. Cyril and Methodius, Nám. J. Herdu 2, 91701 Trnava, Slovakia; [email protected] (R.H.); [email protected] (D.M.); [email protected] (M.M.) 2 Institute of High Mountain Biology, University of Žilina, Univerzitná 8215/1, 01026 Žilina, Slovakia 3 National Agricultural and Food Centre, Research Institute of Plant Production, Bratislavská cesta 122, 92168 Piešt’any, Slovakia 4 INRAE, University Bordeaux, UMR BFP, 33140 Villenave d’Ornon, France; [email protected] 5 Biomedical Research Center of the Slovak Academy of Sciences, Institute of Virology, Dúbravská cesta 9, 84505 Bratislava, Slovakia * Correspondence: [email protected]; Tel.: +421-2-5930-2447 Received: 16 April 2020; Accepted: 22 May 2020; Published: 25 May 2020 Abstract: Plant viruses infecting crop species are causing long-lasting economic losses and are endangering food security worldwide. Ongoing events, such as climate change, changes in agricultural practices, globalization of markets or changes in plant virus vector populations, are affecting plant virus life cycles. Because farmer’s fields are part of the larger environment, the role of wild plant species in plant virus life cycles can provide information about underlying processes during virus transmission and spread. This review focuses on the Solanaceae family, which contains thousands of species growing all around the world, including crop species, wild flora and model plants for genetic research. -

Encyclopaedia of Pests and Natural Enemies in Field Crops Contents Introduction
Encyclopaedia of pests and natural enemies in field crops Contents Introduction Contents Page Integrated pest management Managing pests while encouraging and supporting beneficial insects is an Introduction 2 essential part of an integrated pest management strategy and is a key component of sustainable crop production. Index 3 The number of available insecticides is declining, so it is increasingly important to use them only when absolutely necessary to safeguard their longevity and Identification of larvae 11 minimise the risk of the development of resistance. The Sustainable Use Directive (2009/128/EC) lists a number of provisions aimed at achieving the Pest thresholds: quick reference 12 sustainable use of pesticides, including the promotion of low input regimes, such as integrated pest management. Pests: Effective pest control: Beetles 16 Minimise Maximise the Only use Assess the Bugs and aphids 42 risk by effects of pesticides if risk of cultural natural economically infestation Flies, thrips and sawflies 80 means enemies justified Moths and butterflies 126 This publication Nematodes 150 Building on the success of the Encyclopaedia of arable weeds and the Encyclopaedia of cereal diseases, the three crop divisions (Cereals & Oilseeds, Other pests 162 Potatoes and Horticulture) of the Agriculture and Horticulture Development Board have worked together on this new encyclopaedia providing information Natural enemies: on the identification and management of pests and natural enemies. The latest information has been provided by experts from ADAS, Game and Wildlife Introduction 172 Conservation Trust, Warwick Crop Centre, PGRO and BBRO. Beetles 175 Bugs 181 Centipedes 184 Flies 185 Lacewings 191 Sawflies, wasps, ants and bees 192 Spiders and mites 197 1 Encyclopaedia of pests and natural enemies in field crops Encyclopaedia of pests and natural enemies in field crops 2 Index Index A Acrolepiopsis assectella (leek moth) 139 Black bean aphid (Aphis fabae) 45 Acyrthosiphon pisum (pea aphid) 61 Boettgerilla spp. -

FOCUS Management of Potato Nematodes
J. Hortl. Sci. Vol. 3 (2): 89-106, 2008 FOCUS Management of potato nematodes: An overview K. S. Krishna Prasad1 Central Potato Research Station Ootacamund-643004, Nilgiris District, India E-mail: [email protected] ABSTRACT Root-knot nematodes and cyst nematodes are important constraints that reduce potato yields in India. Three species of Meloidogyne cause root-knots on the crop throughout the country, of which, M. incognita is more wide-spread. Infected tubers also result in marketable-yield-loss particularly in the seed potatoes. The cyst nematodes include two species of Globodera restricted to the hilly regions of Tamil Nadu and are of quarantine importance, inhibiting seed- potato production. Potato produce from these hills is used only for consumption. The endoparasitic nature of their life cycle, deposition of eggs into a gelatinous egg mass in root knot and the female turning in to a hard cyst encompassing the eggs within them in cyst nematode makes them difficult organisms to manage. Both these nematodes exhibit physiologic variation, hence, their management is not absolute with host-resistance. Therefore, an Integrated Nematode Management (INM) is adopted in both the cases. Root-knot nematode in North India is managed using nematode-free seed tubers, crop rotation with maize or wheat and application of 1-2 kg ai /ha Carbofuran 3% G at the time of potato planting. Cyst nematode in Tamil Nadu hills is managed by crop rotation with vegetables, particularly cabbage and carrot, intercropping potato with beans or wheat, alternating nematode resistant potato variety ‘Kufri Swarna’ and application of 2 kg ai /ha Carbofuran 3% G at planting. -

Wisconsin Potato Cyst Nematode (PCN) Survey
Wisconsin Department of Agriculture, Trade and Consumer Protection WWiissccoonnssiinn PPeesstt SSuurrvveeyy RReeppoorrtt POTATO CYST NEMATODE SURVEY Potato cyst nematodes have never been found in Wisconsin. DATCP conducted extensive surveys in 2007 and 2008. Since 2009 PCN testing has been integrated into to University of Wisconsin Seed Potato Certification Program. Wisconsin’s potato industry relies on the certification of potato fields to demonstrate to trading partners that Wisconsin is free from PCN. During the winter of 2006, USDA APHIS initiated an exhaustive nation-wide survey of potato fields for potato cyst nematodes (PCN), after Pale potato cyst nematode was detected in Idaho, and Golden nematode in Quebec, Canada earlier that year. These two potato pests, the pale potato cyst nematode (Globodera pallida) and the Golden nematode (Globodera rostochiensis) are economically significant quarantine pests. They are microscopically small, worm-like creatures, whose females form durable pinhead-sized cysts that are filled with eggs (Figure2). They can survive in the soil for decades and still infect potatoes. These potato cyst nematodes feed on the roots of potatoes, tomatoes and eggplants. Widespread in Europe and South America, PCN were only known to occur in few locations in North America: Pale potato cyst nematode in Newfoundland, CD, and Golden nematode in parts of British Columbia and Newfoundland, CD, and New York State. In Wisconsin the 2007 survey collected 1,808 samples from 8625 acres of seed potato fields and a small subsample from 66,000 acres of potatoes grown for consumption in a total of 42 counties (Figure 1). In 2008, 610 soil samples were collected from 3050 Figure 1.