Identification and Prevalence of Potato Cyst Nematodes and Root-Knot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
A Revision of the Family Heteroderidae (Nematoda: Tylenchoidea) I
A REVISION OF THE FAMILY HETERODERIDAE (NEMATODA: TYLENCHOIDEA) I. THE FAMILY HETERODERIDAE AND ITS SUBFAMILIES BY W. M. WOUTS Entomology Division, Department of Scientific and Industrial Research, Nelson, New Zealand The family Heteroderidae and the subfamilies Heteroderinae and Meloidoderinae are redefined. The subfamily Meloidogyninae is raised to family Meloidogynidae. The genus Meloidoderita Poghossian, 1966 is transferred to the family Meloidogynidae.Ataloderinae n. subfam. is proposed and diagnosed in the family Heteroderidae. A key to the three subfamilies is presented and a possible phylogeny of the family Heteroderidae is discussed. The family Heteroderidae (Filipjev & Schuurmans Stekhoven, 1941) Skar- bilovich, 1947 was proposed for the sexually dimorphic, obligate plant parasites of the nematode genera Heterodera Schmidt, 1871 and T'ylenchulu.r Cobb, 1913. Because of differences in body length of the female, number of ovaries, position of the excretory pore and presence or apparent absence of the anal opening they were placed in separate subfamilies; Heteroderinae Filipjev & Schuurmans Stek- hoven, 1941 and Tylenchulinae Skarbilovich, 1947. Independently Thorne (1949), on the basis of sexual dimorphism, proposed Heteroderidae to include Heterodera and Meloidogyne Goeldi, 1892; he considered the short rounded tail of the male and the absence of caudal alae as family charac- ters, and included Heteroderinae as the only subfamily. Chitwood & Chitwood ( 1950) ignored sexual dimorphism and based the family on the heavy stylet and general characters of the head and the oesophagus of the adults. They recognised as subfamilies Heteroderinae, Hoplolaiminae Filipjev, 1934 and the new subfamily Nacobbinae. Skarbilovich (1959) re-emphasized sexual dimorphism as a family character and stated that "It is quite illegitimate for the [previous] authors to assign the subfamily Hoplolaiminae to the family Heteroderidae". -

Pest Management Strategic Plan for Organic Potato Production in the West
Pest Management Strategic Plan for Organic Potato Production in the West Summary of workshops held on February 16, 2006 Buhl, Idaho and January 9, 2008 Portland, Oregon Issue Date December 19, 2008 Lead Authors: Jennifer Miller, Ronda Hirnyck, Lisa Downey-Blecker Editor: Diane Clarke This project was sponsored by the Western Integrated Pest Management Center, which is funded by the United States Department of Agriculture, Cooperative State Research, Education, and Extension Service. Additional funding was provided by the Organic Farming Research Foundation and the Bullitt Foundation. Table of Contents Work Group .........................................................................................................................3 Summary of the Most Critical Needs in Organic Potato Production in the West ................5 Introduction ..........................................................................................................................6 Production Overview .........................................................................................................11 California ...............................................................................................................11 Colorado .................................................................................................................12 Columbia Basin ......................................................................................................12 Idaho ......................................................................................................................13 -

PCN Guidelines, and Potato Cyst Nematodes (Globodera Rostochiensis Or Globodera Pallida) Were Not Detected.”
Canada and United States Guidelines on Surveillance and Phytosanitary Actions for the Potato Cyst Nematodes Globodera rostochiensis and Globodera pallida 7 May 2014 Table of Contents 1. Introduction ...........................................................................................................................................................3 2. Rationale for phytosanitary actions ........................................................................................................................3 3. Soil sampling and laboratory analysis procedures .................................................................................................4 4. Phytosanitary measures ........................................................................................................................................4 5. Regulated articles .................................................................................................................................................5 6. National PCN detection survey..............................................................................................................................6 7. Pest-free places of production or pest-free production sites within regulated areas ...............................................6 8. Phytosanitary certification of seed potatoes ..........................................................................................................7 9. Releasing land from regulatory control ..................................................................................................................8 -

Plant-Parasitic Nematodes and Their Management: a Review
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by International Institute for Science, Technology and Education (IISTE): E-Journals Journal of Biology, Agriculture and Healthcare www.iiste.org ISSN 2224-3208 (Paper) ISSN 2225-093X (Online) Vol.8, No.1, 2018 Plant-Parasitic Nematodes and Their Management: A Review Misgana Mitiku Department of Plant Pathology, Southern Agricultural Research Institute, Jinka, Agricultural Research Center, Jinka, Ethiopia Abstract Nowhere will the need to sustainably increase agricultural productivity in line with increasing demand be more pertinent than in resource poor areas of the world, especially Africa, where populations are most rapidly expanding. Although a 35% population increase is projected by 2050. Significant improvements are consequently necessary in terms of resource use efficiency. In moving crop yields towards an efficiency frontier, optimal pest and disease management will be essential, especially as the proportional production of some commodities steadily shifts. With this in mind, it is essential that the full spectrums of crop production limitations are considered appropriately, including the often overlooked nematode constraints about half of all nematode species are marine nematodes, 25% are free-living, soil inhabiting nematodes, I5% are animal and human parasites and l0% are plant parasites. Today, even with modern technology, 5-l0% of crop production is lost due to nematodes in developed countries. So, the aim of this work was to review some agricultural nematodes genera, species they contain and their management methods. In this review work the species, feeding habit, morphology, host and symptoms they show on the effected plant and management of eleven nematode genera was reviewed. -

Theory Manual Course No. Pl. Path
NAVSARI AGRICULTURAL UNIVERSITY Theory Manual INTRODUCTORY PLANT NEMATOLOGY Course No. Pl. Path 2.2 (V Dean’s) nd 2 Semester B.Sc. (Hons.) Agri. PROF.R.R.PATEL, ASSISTANT PROFESSOR Dr.D.M.PATHAK, ASSOCIATE PROFESSOR Dr.R.R.WAGHUNDE, ASSISTANT PROFESSOR DEPARTMENT OF PLANT PATHOLOGY COLLEGE OF AGRICULTURE NAVSARI AGRICULTURAL UNIVERSITY BHARUCH 392012 1 GENERAL INTRODUCTION What are the nematodes? Nematodes are belongs to animal kingdom, they are triploblastic, unsegmented, bilateral symmetrical, pseudocoelomateandhaving well developed reproductive, nervous, excretoryand digestive system where as the circulatory and respiratory systems are absent but govern by the pseudocoelomic fluid. Plant Nematology: Nematology is a science deals with the study of morphology, taxonomy, classification, biology, symptomatology and management of {plant pathogenic} nematode (PPN). The word nematode is made up of two Greek words, Nema means thread like and eidos means form. The words Nematodes is derived from Greek words ‘Nema+oides’ meaning „Thread + form‟(thread like organism ) therefore, they also called threadworms. They are also known as roundworms because nematode body tubular is shape. The movement (serpentine) of nematodes like eel (marine fish), so also called them eelworm in U.K. and Nema in U.S.A. Roundworms by Zoologist Nematodes are a diverse group of organisms, which are found in many different environments. Approximately 50% of known nematode species are marine, 25% are free-living species found in soil or freshwater, 15% are parasites of animals, and 10% of known nematode species are parasites of plants (see figure at left). The study of nematodes has traditionally been viewed as three separate disciplines: (1) Helminthology dealing with the study of nematodes and other worms parasitic in vertebrates (mainly those of importance to human and veterinary medicine). -

Biology and Control of the Anguinid Nematode
BIOLOGY AND CONTROL OF THE AIIGTIINID NEMATODE ASSOCIATED WITH F'LOOD PLAIN STAGGERS by TERRY B.ERTOZZI (B.Sc. (Hons Zool.), University of Adelaide) Thesis submitted for the degree of Doctor of Philosophy in The University of Adelaide (School of Agriculture and Wine) September 2003 Table of Contents Title Table of contents.... Summary Statement..... Acknowledgments Chapter 1 Introduction ... Chapter 2 Review of Literature 2.I Introduction.. 4 2.2 The 8acterium................ 4 2.2.I Taxonomic status..' 4 2.2.2 The toxins and toxin production.... 6 2.2.3 Symptoms of poisoning................. 7 2.2.4 Association with nematodes .......... 9 2.3 Nematodes of the genus Anguina 10 2.3.1 Taxonomy and sYstematics 10 2.3.2 Life cycle 13 2.4 Management 15 2.4.1 Identifi cation...................'..... 16 2.4.2 Agronomicmethods t6 2.4.3 FungalAntagonists l7 2.4.4 Other strategies 19 2.5 Conclusions 20 Chapter 3 General Methods 3.1 Field sites... 22 3.2 Collection and storage of Polypogon monspeliensis and Agrostis avenaceø seed 23 3.3 Surface sterilisation and germination of seed 23 3.4 Collection and storage of nematode galls .'.'.'.....'.....' 24 3.5 Ext¡action ofjuvenile nematodes from galls 24 3.6 Counting nematodes 24 3.7 Pot experiments............. 24 Chapter 4 Distribution of Flood Plain Staggers 4.1 lntroduction 26 4.2 Materials and Methods..............'.. 27 4.2.1 Survey of Murray River flood plains......... 27 4.2.2 Survey of southeastern South Australia .... 28 4.2.3 Surveys of northern New South Wales...... 28 4.3 Results 29 4.3.1 Survey of Murray River flood plains... -
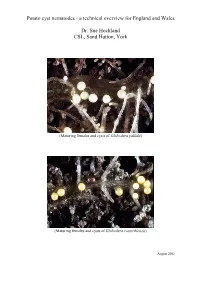
Potato Cyst Nematodes - a Technical Overview for England and Wales
Potato cyst nematodes - a technical overview for England and Wales Dr. Sue Hockland CSL, Sand Hutton, York (Maturing females and cysts of Globodera pallida) (Maturing females and cysts of Globodera rostochiensis) August 2002 Contents Page • Executive Summary 1 • Introduction 2 • PCN: species and diagnosis 2 • Biology of PCN species 3 • Detection 4 • Host plants of PCN 4 • Symptoms 5 • Damage 5 • Pathotypes and Host Plant Resistance 5 • Distribution and spread in England and Wales 6 • Distribution in Europe and elsewhere 9 • Statutory Management of PCN 11 • Management of PCN in ware potatoes 13 • Chemical methods 14 • Non-chemical methods 14 • Conclusion 16 • Acknowledgements 17 Executive Summary The pests Potato cyst nematode (PCN) is the name commonly given to two species of cyst nematode that attack potato, namely Globodera pallida (Stone) Behrens and G. rostochiensis (Wollenweber) Behrens. They are two of the most important pests of potato in England and Wales, feeding on potato roots, causing losses of yield and costs that vary and are difficult to estimate. Published papers usually quote losses of about 9% of annual yield, estimated at about £43 million for the UK, based on the mean value of the crop from 1990-1995. Adaptations to a plant-parasitic life In both species the female forms a hard covering around her eggs when she dies, creating a ‘cyst’ which protects the eggs and developing juveniles from desiccation, predation and chemical control. Only a proportion of the eggs hatch from the cyst each year, and in G. pallida this occurs at a slower rate and with a later annual peak of hatching than G. -

Note 20: List of Plant-Parasitic Nematodes Found in North Carolina
nema note 20 NCDA&CS AGRONOMIC DIVISION NEMATODE ASSAY SECTION PHYSICAL ADDRESS 4300 REEDY CREEK ROAD List of Plant-Parasitic Nematodes Recorded in RALEIGH NC 27607-6465 North Carolina MAILING ADDRESS 1040 MAIL SERVICE CENTER Weimin Ye RALEIGH NC 27699-1040 PHONE: 919-733-2655 FAX: 919-733-2837 North Carolina's agricultural industry, including food, fiber, ornamentals and forestry, contributes $84 billion to the state's annual economy, accounts for more than 17 percent of the state's DR. WEIMIN YE NEMATOLOGIST income, and employs 17 percent of the work force. North Carolina is one of the most diversified agricultural states in the nation. DR. COLLEEN HUDAK-WISE Approximately, 50,000 farmers grow over 80 different commodities in DIVISION DIRECTOR North Carolina utilizing 8.2 million of the state's 12.5 million hectares to furnish consumers a dependable and affordable supply STEVE TROXLER of food and fiber. North Carolina produces more tobacco and sweet AGRICULTURE COMMISSIONER potatoes than any other state, ranks second in Christmas tree and third in tomato production. The state ranks nineth nationally in farm cash receipts of over $10.8 billion (NCDA&CS Agricultural Statistics, 2017). Plant-parasitic nematodes are recognized as one of the greatest threat to crops throughout the world. Nematodes alone or in combination with other soil microorganisms have been found to attack almost every part of the plant including roots, stems, leaves, fruits and seeds. Crop damage caused worldwide by plant nematodes has been estimated at $US80 billion per year (Nicol et al., 2011). All crops are damaged by at least one species of nematode. -

Phylogenetic Implications of Phasmid Absence in Males of Three Genera in Heteroderinae 1 L
Journal of Nematology 22(3):386-394. 1990. © The Society of Nematologists 1990. Phylogenetic Implications of Phasmid Absence in Males of Three Genera in Heteroderinae 1 L. K. CARTA2 AND J. G. BALDWINs Abstract: Absence of the phasmid was demonstrated with the transmission electron microscope in immature third-stage (M3) and fourth-stage (M4) males and mature fifth-stage males (M5) of Heterodera schachtii, M3 and M4 of Verutus volvingentis, and M5 of Cactodera eremica. This absence was supported by the lack of phasmid staining with Coomassie blue and cobalt sulfide. All phasmid structures, except the canal and ampulla, were absent in the postpenetration second-stagejuvenile (]2) of H. schachtii. The prepenetration V. volvingentis J2 differs from H. schachtii by having only a canal remnant and no ampulla. This and parsimonious evidence suggest that these two types of phasmids probably evolved in parallel, although ampulla and receptor cavity shape are similar. Absence of the male phasmid throughout development might be associated with an amphimictic mode of reproduction. Phasmid function is discussed, and female pheromone reception ruled out. Variations in ampulla shape are evaluated as phylogenetic character states within the Heteroderinae and putative phylogenetic outgroup Hoplolaimidae. Key words: anaphimixis, ampulla, cell death, Cactodera eremica, Heterodera schachtii, Heteroderinae, parallel evolution, parthenogenesis, phasmid, phylogeny, ultrastructure, Verutus volvingentis. Phasmid sensory organs on the tails of phasmid openings in the males of most gen- secernentean nematodes are sometimes era within the plant-parasitic Heteroderi- notoriously difficult to locate with the light nae, except Meloidodera (24) and perhaps microscope (18). Because the assignment Cryphodera (10) and Zelandodera (43). -

Vittatidera Zeaphila (Nematoda: Heteroderidae), a New Genus and Species of Cyst Nematode Parasitic on Corn (Zea Mays)
Journal of Nematology 42(2):139–150. 2010. Ó The Society of Nematologists 2010. Vittatidera zeaphila (Nematoda: Heteroderidae), a new genus and species of cyst nematode parasitic on corn (Zea mays) 1 2 3 4 5 ERNEST C. BERNARD, ZAFAR A. HANDOO, THOMAS O. POWERS, PATRICIA A. DONALD, ROBERT D. HEINZ Abstract: A new genus and species of cyst nematode, Vittatidera zeaphila, is described from Tennessee. The new genus is superficially similar to Cactodera but is distinguished from other cyst-forming taxa in having a persistent lateral field in females and cysts, persistent vulval lips covering a circumfenestrate vulva, and subventral gland nuclei of the female contained in a separate small lobe. Infective juveniles (J2) are distinguished from all previously described Cactodera spp. by the short stylet in the second-stage juvenile (14-17 mm); J2 of Cactodera spp. have stylets at least 18 mm long. The new species also is unusual in that the females produce large egg masses. Known hosts are corn and goosegrass. DNA analysis suggests that Vittatidera forms a separate group apart from other cyst-forming genera within Heteroderinae. Key words: cyst nematode, Eleusine indica, goosegrass, maize, molecular analysis, new genus, taxonomy, Vittatidera zeaphila, Zea mays. Cyst nematodes are widespread but with the excep- processed to glycerin with a rapid method (Seinhorst tions of Heterodera avenae Wollenweber and H. zeae Koshi, 1959), and mounted in anhydrous glycerin on micro- Swarup & Sethi (Baldwin & Mundo-Ocampo 1991) are scope slides. not significant parasites of Poaceae. In the late 1970s the DNA analyses Juvenile nematodes of Vittatidera zeaphila first author collected specimens of a cyst nematode from were obtained from culture. -

ÜRETİM ALANLARINDA KÖK-UR NEMATODLARI (Meloidogyne Spp.)’NIN DURUMU
i EGE ÜNİVERSİTESİ FEN BİLİMLERİ ENSTİTÜSÜ (YÜKSEK LİSANS TEZİ) ÖDEMİŞ VE KİRAZ (İZMİR) İLÇELERİNDE TURŞULUK HIYAR (Cucumis sativus L.) ÜRETİM ALANLARINDA KÖK-UR NEMATODLARI (Meloidogyne spp.)’NIN DURUMU Esmeray CAFARLI Tez Danışmanı : Doç. Dr. Galip KAŞKAVALCI Bitki Koruma Anabilim Dalı Bilim Dalı Kodu : 501.02.01 Sunuş Tarihi: 12.02.2013 Bornova-İZMİR 2013 ii iii Esmeray CAFARLI tarafından Yüksek Lisans tezi olarak sunulan “Ödemiş ve Kiraz (İzmir) İlçelerinde Turşuluk Hıyar (Cucumis sativus L.) Üretim Alanlarında Kök-Ur Nematodları (Meloidogyne spp.)’nın Durumu” baĢlıklı bu çalıĢma E.Ü. Lisansüstü Eğitim ve Öğretim Yönergesi’nin ilgili hükümleri uyarınca tarafımızdan değerlendirilerek savunmaya değer bulunmuĢ ve 12/02/2013 tarihinde yapılan tez savunma sınavında aday oybirliği/oyçokluğu ile baĢarılı bulunmuĢtur. Jüri üyeleri: İmza Jüri Başkanı : Doç. Dr. Galip KAġKAVALCI ……………………. Raportör üye : Doç. Dr. Ferit TURANLI ……………………. Üye : Prof. Dr. Dursun Eġ ĠYOK ……………………. iv v ÖZET ÖDEMİŞ VE KİRAZ (İZMİR) İLÇELERİNDE TURŞULUK HIYAR (Cucumis sativus L.) ÜRETİM ALANLARINDA KÖK-UR NEMATODLARI (Meloidogyne spp.)’NIN DURUMU CAFARLI, Esmeray Yüksek Lisans Tezi, Bitki Koruma Anabilim Dalı Tez Yöneticisi: Doç. Dr. Galip KAġKAVALCI ġubat 2013, 66 sayfa Bu çalıĢmanın amacı, Ġzmir Ġli ÖdemiĢ ve Kiraz ilçeleri turĢuluk hıyar üretimi yapılan tarlalarda bulunan kök-ur nematodlarının yayılıĢ ve bulaĢıklık oranları ile türlerinin saptanmasıdır. AraĢtırmanın materyalini, ÖdemiĢ ve Kiraz ilçeleri tarlarından alınan, kök-ur nematodu ile bulaĢık bitki materyali oluĢturmuĢtur. ÖdemiĢ Ġlçesi’nde 13 köydeki 62 tarladan, Kiraz Ġlçesi’nde ise 11 köydeki 34 tarladan bitki kök örnekleri alınıp, kökler 0-10 bulaĢıklık ıskalasına göre değerlendirilmiĢtir. YayılıĢ ve bulaĢıklık tespiti çalıĢmaları neticesinde; kök-ur nematodları ile bulaĢıklığın ÖdemiĢ Ġlçesi tarlarında % 17,74, Kiraz’da ise % 17,65 oranlarında olduğu saptanmıĢtır. -

Pale Cyst Nematode Globodera Pallida
Michigan State University’s invasive species factsheets Pale cyst nematode Globodera pallida The pale cyst nematode is a serious pest of potatoes around the world and is a target of strict regulatory actions in the United States. An introduction to Michigan may adversely affect production and marketing of potatoes and other solanaceous crops. Michigan risk maps for exotic plant pests. Other common names potato cyst nematode, white potato cyst nematode, pale potato cyst nematode Systematic position Nematoda > Tylenchida > Heteroderidae > Globodera pallida (Stone) Behrens Global distribution Worldwide distribution in potato-producing regions. Mature females (white) and a cyst (brown) of potato cyst nematode attached to the roots. (Photo: B. Hammeraas, Bioforsk - Norwegian Institute for Agricultural Africa: Algeria, Tunisia; Asia: India, Pakistan, and Environmental Research, Bugwood.org) Turkey; Europe: Austria, Belgium, Bulgaria, Croatia, Czech Republic, Cyprus, Faroe Islands, Finland, pale cyst nematode move to, penetrate and feed on host France, Germany, Greece, Hungary, Iceland, Ireland, roots. After mating, fertilized eggs develop inside females Italy, Luxembourg, Malta, Netherlands, Norway, Poland, that are attached to roots. When females die their skin Portugal, Romania, Spain, Sweden, Switzerland, United hardens and becomes a protective brown cover (cyst) Kingdom; Latin America: Argentina, Bolivia, Chile, around the eggs. Each cyst contains hundreds of eggs, and Colombia, Ecuador, Panama, Peru, Venezuela; Oceania: it can remain viable for many years in the absence of host New Zealand; North America: Idaho, Canada (a small plants. One generation normally occurs during one growing area of Newfoundland). season. Quarantine status Identification In 2006, the pale cyst nematode was detected at At flowering or later stages of a host plant, mature a potato processing facility in Idaho.