University of Dundee DOCTOR of PHILOSOPHY Population Dynamics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pest Management Strategic Plan for Organic Potato Production in the West
Pest Management Strategic Plan for Organic Potato Production in the West Summary of workshops held on February 16, 2006 Buhl, Idaho and January 9, 2008 Portland, Oregon Issue Date December 19, 2008 Lead Authors: Jennifer Miller, Ronda Hirnyck, Lisa Downey-Blecker Editor: Diane Clarke This project was sponsored by the Western Integrated Pest Management Center, which is funded by the United States Department of Agriculture, Cooperative State Research, Education, and Extension Service. Additional funding was provided by the Organic Farming Research Foundation and the Bullitt Foundation. Table of Contents Work Group .........................................................................................................................3 Summary of the Most Critical Needs in Organic Potato Production in the West ................5 Introduction ..........................................................................................................................6 Production Overview .........................................................................................................11 California ...............................................................................................................11 Colorado .................................................................................................................12 Columbia Basin ......................................................................................................12 Idaho ......................................................................................................................13 -

PCN Guidelines, and Potato Cyst Nematodes (Globodera Rostochiensis Or Globodera Pallida) Were Not Detected.”
Canada and United States Guidelines on Surveillance and Phytosanitary Actions for the Potato Cyst Nematodes Globodera rostochiensis and Globodera pallida 7 May 2014 Table of Contents 1. Introduction ...........................................................................................................................................................3 2. Rationale for phytosanitary actions ........................................................................................................................3 3. Soil sampling and laboratory analysis procedures .................................................................................................4 4. Phytosanitary measures ........................................................................................................................................4 5. Regulated articles .................................................................................................................................................5 6. National PCN detection survey..............................................................................................................................6 7. Pest-free places of production or pest-free production sites within regulated areas ...............................................6 8. Phytosanitary certification of seed potatoes ..........................................................................................................7 9. Releasing land from regulatory control ..................................................................................................................8 -
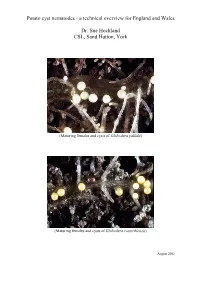
Potato Cyst Nematodes - a Technical Overview for England and Wales
Potato cyst nematodes - a technical overview for England and Wales Dr. Sue Hockland CSL, Sand Hutton, York (Maturing females and cysts of Globodera pallida) (Maturing females and cysts of Globodera rostochiensis) August 2002 Contents Page • Executive Summary 1 • Introduction 2 • PCN: species and diagnosis 2 • Biology of PCN species 3 • Detection 4 • Host plants of PCN 4 • Symptoms 5 • Damage 5 • Pathotypes and Host Plant Resistance 5 • Distribution and spread in England and Wales 6 • Distribution in Europe and elsewhere 9 • Statutory Management of PCN 11 • Management of PCN in ware potatoes 13 • Chemical methods 14 • Non-chemical methods 14 • Conclusion 16 • Acknowledgements 17 Executive Summary The pests Potato cyst nematode (PCN) is the name commonly given to two species of cyst nematode that attack potato, namely Globodera pallida (Stone) Behrens and G. rostochiensis (Wollenweber) Behrens. They are two of the most important pests of potato in England and Wales, feeding on potato roots, causing losses of yield and costs that vary and are difficult to estimate. Published papers usually quote losses of about 9% of annual yield, estimated at about £43 million for the UK, based on the mean value of the crop from 1990-1995. Adaptations to a plant-parasitic life In both species the female forms a hard covering around her eggs when she dies, creating a ‘cyst’ which protects the eggs and developing juveniles from desiccation, predation and chemical control. Only a proportion of the eggs hatch from the cyst each year, and in G. pallida this occurs at a slower rate and with a later annual peak of hatching than G. -

Pale Cyst Nematode Globodera Pallida
Michigan State University’s invasive species factsheets Pale cyst nematode Globodera pallida The pale cyst nematode is a serious pest of potatoes around the world and is a target of strict regulatory actions in the United States. An introduction to Michigan may adversely affect production and marketing of potatoes and other solanaceous crops. Michigan risk maps for exotic plant pests. Other common names potato cyst nematode, white potato cyst nematode, pale potato cyst nematode Systematic position Nematoda > Tylenchida > Heteroderidae > Globodera pallida (Stone) Behrens Global distribution Worldwide distribution in potato-producing regions. Mature females (white) and a cyst (brown) of potato cyst nematode attached to the roots. (Photo: B. Hammeraas, Bioforsk - Norwegian Institute for Agricultural Africa: Algeria, Tunisia; Asia: India, Pakistan, and Environmental Research, Bugwood.org) Turkey; Europe: Austria, Belgium, Bulgaria, Croatia, Czech Republic, Cyprus, Faroe Islands, Finland, pale cyst nematode move to, penetrate and feed on host France, Germany, Greece, Hungary, Iceland, Ireland, roots. After mating, fertilized eggs develop inside females Italy, Luxembourg, Malta, Netherlands, Norway, Poland, that are attached to roots. When females die their skin Portugal, Romania, Spain, Sweden, Switzerland, United hardens and becomes a protective brown cover (cyst) Kingdom; Latin America: Argentina, Bolivia, Chile, around the eggs. Each cyst contains hundreds of eggs, and Colombia, Ecuador, Panama, Peru, Venezuela; Oceania: it can remain viable for many years in the absence of host New Zealand; North America: Idaho, Canada (a small plants. One generation normally occurs during one growing area of Newfoundland). season. Quarantine status Identification In 2006, the pale cyst nematode was detected at At flowering or later stages of a host plant, mature a potato processing facility in Idaho. -

Potato Cyst Nematodes Factsheet
Biosecurity SA – Plant Health Exotic Plant Pest Hotline: 1800 084 881 (available 24 hours) Email [email protected] June 2017 Potato cyst nematodes Globodera pallida and Globodera rostochiensis WHAT IS IT? Pale potato cyst nematode (Globodera pallida) is an exotic plant pest not present in Australia Golden potato cyst nematode (Globodera rostochiensis) is present in some areas of Victoria but is not in South Australia. These nematodes are a serious threat to Australia’s potato industry. Potato cyst nematodes (PCN) are soil-borne microscopic worms which feed on roots of potato plants. Root development and tuber yield is reduced and plant growth is stunted. The biology and symptoms caused by both species Crop damage caused by PCN. are similar. Photo courtesy© Syngenta 2013 The occurrence of PCN is related to the presence of a host and not to soil type or soil temperature. The preferred host of PCN is potato. PCN can infest plants such as tomato, eggplant and some solanaceous weeds. PCN can survive as cysts in the soil for many years in the absence of host plants. PCN is subject to stringent quarantine and regulatory procedures wherever it occurs. WHAT TO LOOK FOR? The symptoms of attack by Globodera species are not specific. Symptoms may appear similar to water or nutrient deficiencies or wilt diseases. Infested potato plants have a reduced root system which is abnormally branched and brownish in colour. Growth is stunted, leaves yellow early or turn a dull colour, flowering is delayed and plants may wilt. At or after flowering very tiny white, yellow or brown cysts about the size of a pin head (0.5 mm) might be seen on the outside of roots HOW DOES IT SURVIVE? PCN are small worms less than 1 mm in size. -

Encyclopaedia of Pests and Natural Enemies in Field Crops Contents Introduction
Encyclopaedia of pests and natural enemies in field crops Contents Introduction Contents Page Integrated pest management Managing pests while encouraging and supporting beneficial insects is an Introduction 2 essential part of an integrated pest management strategy and is a key component of sustainable crop production. Index 3 The number of available insecticides is declining, so it is increasingly important to use them only when absolutely necessary to safeguard their longevity and Identification of larvae 11 minimise the risk of the development of resistance. The Sustainable Use Directive (2009/128/EC) lists a number of provisions aimed at achieving the Pest thresholds: quick reference 12 sustainable use of pesticides, including the promotion of low input regimes, such as integrated pest management. Pests: Effective pest control: Beetles 16 Minimise Maximise the Only use Assess the Bugs and aphids 42 risk by effects of pesticides if risk of cultural natural economically infestation Flies, thrips and sawflies 80 means enemies justified Moths and butterflies 126 This publication Nematodes 150 Building on the success of the Encyclopaedia of arable weeds and the Encyclopaedia of cereal diseases, the three crop divisions (Cereals & Oilseeds, Other pests 162 Potatoes and Horticulture) of the Agriculture and Horticulture Development Board have worked together on this new encyclopaedia providing information Natural enemies: on the identification and management of pests and natural enemies. The latest information has been provided by experts from ADAS, Game and Wildlife Introduction 172 Conservation Trust, Warwick Crop Centre, PGRO and BBRO. Beetles 175 Bugs 181 Centipedes 184 Flies 185 Lacewings 191 Sawflies, wasps, ants and bees 192 Spiders and mites 197 1 Encyclopaedia of pests and natural enemies in field crops Encyclopaedia of pests and natural enemies in field crops 2 Index Index A Acrolepiopsis assectella (leek moth) 139 Black bean aphid (Aphis fabae) 45 Acyrthosiphon pisum (pea aphid) 61 Boettgerilla spp. -

FOCUS Management of Potato Nematodes
J. Hortl. Sci. Vol. 3 (2): 89-106, 2008 FOCUS Management of potato nematodes: An overview K. S. Krishna Prasad1 Central Potato Research Station Ootacamund-643004, Nilgiris District, India E-mail: [email protected] ABSTRACT Root-knot nematodes and cyst nematodes are important constraints that reduce potato yields in India. Three species of Meloidogyne cause root-knots on the crop throughout the country, of which, M. incognita is more wide-spread. Infected tubers also result in marketable-yield-loss particularly in the seed potatoes. The cyst nematodes include two species of Globodera restricted to the hilly regions of Tamil Nadu and are of quarantine importance, inhibiting seed- potato production. Potato produce from these hills is used only for consumption. The endoparasitic nature of their life cycle, deposition of eggs into a gelatinous egg mass in root knot and the female turning in to a hard cyst encompassing the eggs within them in cyst nematode makes them difficult organisms to manage. Both these nematodes exhibit physiologic variation, hence, their management is not absolute with host-resistance. Therefore, an Integrated Nematode Management (INM) is adopted in both the cases. Root-knot nematode in North India is managed using nematode-free seed tubers, crop rotation with maize or wheat and application of 1-2 kg ai /ha Carbofuran 3% G at the time of potato planting. Cyst nematode in Tamil Nadu hills is managed by crop rotation with vegetables, particularly cabbage and carrot, intercropping potato with beans or wheat, alternating nematode resistant potato variety ‘Kufri Swarna’ and application of 2 kg ai /ha Carbofuran 3% G at planting. -

Wisconsin Potato Cyst Nematode (PCN) Survey
Wisconsin Department of Agriculture, Trade and Consumer Protection WWiissccoonnssiinn PPeesstt SSuurrvveeyy RReeppoorrtt POTATO CYST NEMATODE SURVEY Potato cyst nematodes have never been found in Wisconsin. DATCP conducted extensive surveys in 2007 and 2008. Since 2009 PCN testing has been integrated into to University of Wisconsin Seed Potato Certification Program. Wisconsin’s potato industry relies on the certification of potato fields to demonstrate to trading partners that Wisconsin is free from PCN. During the winter of 2006, USDA APHIS initiated an exhaustive nation-wide survey of potato fields for potato cyst nematodes (PCN), after Pale potato cyst nematode was detected in Idaho, and Golden nematode in Quebec, Canada earlier that year. These two potato pests, the pale potato cyst nematode (Globodera pallida) and the Golden nematode (Globodera rostochiensis) are economically significant quarantine pests. They are microscopically small, worm-like creatures, whose females form durable pinhead-sized cysts that are filled with eggs (Figure2). They can survive in the soil for decades and still infect potatoes. These potato cyst nematodes feed on the roots of potatoes, tomatoes and eggplants. Widespread in Europe and South America, PCN were only known to occur in few locations in North America: Pale potato cyst nematode in Newfoundland, CD, and Golden nematode in parts of British Columbia and Newfoundland, CD, and New York State. In Wisconsin the 2007 survey collected 1,808 samples from 8625 acres of seed potato fields and a small subsample from 66,000 acres of potatoes grown for consumption in a total of 42 counties (Figure 1). In 2008, 610 soil samples were collected from 3050 Figure 1. -

Genetic Diversity of the Potato Cyst Nematode in the Netherlands Promotor: Dr
Genetic diversity of the potato cyst nematode in the Netherlands Promotor: dr. R.W. Goldbach, hoogleraar in de Virologie Co-promotoren: dr.ir. F.J. Gommers, universitair hoofddocent bij de vakgroep Nematologie, Landbouwuniversiteit Wageningen dr.ir. J. Bakker, universitair hoofddocent bij de vakgroep Nematologie, Landbouwuniversiteit Wageningen Genetic diversity of the potato cyst nematode in the Netherlands RoelofTjall o Folkertsma Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van de Landbouwuniversiteit te Wageningen, dr. C.M. Karssen, in het openbaar te verdedigen op vrijdag 10 oktober 1997 des namiddags te 16.00 uur in de Aula. / GENETIC DIVERSITY OF THE POTATO CYST NEMATODE IN THE NETHERLANDS ISBN 90-5485-748-x Vormgeving binnenwerk en omslag: Nynke Folkertsma Corps 3 op twee BNO 's Gravenhage Druk: Ponsen & Looijen BV Wageningen BIBLIOTHEEK LA N! DBOUWUNIVERSITFT T V.'AGENINGEN fONQ$?Q\ 2.5^ö Stellingen 1. In tegenstelling tot wat algemeen wordt aangenomen is de resistentieveredeling tegen het aardappelcystenaaltje niet een op voorhand verloren wapenwedloop. dit proefschrift 2. De aardappelcystenaaltje s Globodera rostochiensis en G. pallida hebben Nederland onafhankelijk van elkaar gekoloniseerd. dit proefschrift 3. De aardappelzetmeelindustrie heeft een significante bijdrage geleverd aan de variatie binnen en tussen populaties van het aardappelcystenaaltje in Noord-Oost Nederland. dit proefschrift 4. Nu het verzadigen van genetische kaarten met DNA markers onder handbereik ligt, wordt de bottleneck in het veredelingsonderzoek het ontwerpen van betrouwbare 'groene' experimenten. 5. Uitgaande vanHaeckel' s definitie vanfylogeni e ishe tonjuis t eenfylogenetisch e boom van DNA sequenties te presenteren zonder deze vanuit evolutionair oogpunt te interpreteren. -

Identification and Prevalence of Potato Cyst Nematodes and Root-Knot
Türk. entomol. derg., 2020, 44 (2): 259-272 ISSN 1010-6960 DOI: http://dx.doi.org/10.16970/entoted.683173 E-ISSN 2536-491X Original article (Orijinal araştırma) Identification and prevalence of potato cyst nematodes and root-knot nematodes in the potato production areas of İzmir Province, Turkey1 İzmir İli (Türkiye) patates üretim alanlarında patates kist nematodları ve kök-ur nematodlarının teşhisi ve yaygınlığı Hülya DEMİRBAŞ PEHLİVAN2* Galip KAŞKAVALCI3 Ece B. KASAPOĞLU ULUDAMAR4 Halil TOKTAY5 İ. Halil ELEKCİOĞLU4 Abstract Globodera spp.and Meloidogyne spp. were identified using morphological and molecular methods. Also, the distribution and population densities of these nematodes were determined in potato cultivation areas of İzmir (Turkey) in 2015. Two hundred and twenty-three soil samples were collected during the survey and 32 samples were found to be infested with Globodera spp. and 41 samples with Meloidogyne spp. The identification of nematodes was made morphologically using perennial patterns of cyst/female individuals and morphometrics of second stage juveniles. Also, species specific primers were used for molecular identification. Only Globodera rostochiensis (Wollenweber, 1923) Skarbilovich, 1959 (Tylenchida: Heteroderidae) were found in the samples that contained cyst nematodes. Also, the root-knot nematodes, Meloidogyne chitwoodi Golden, O'Bannon, Santo & Finley, 1980, Meloidogyne hapla (Chitwood, 1949), Meloidogyne incognita (Kofoid & White, 1919) Chitwood, 1949, and Meloidogyne javanica (Treub, 1885) Chitwood, 1949 (Tylenchida: Meloidogynidae) were found. The prevalence rates of Meloidogyne species were determined as 2.4, 12.2, 61.0 and 24.4%, respectively. In terms of the number of individuals in soil, all G. rostochiensis (10 eggs/g soil) and M. chitwoodi (1 juvenile/250 cm3 soil) population levels were detected above the economic damage thresholds for potato production. -

Parasitic Nematodes and Rhizoctonia Solani in Potato Fields
Department of Ecology Interactions between some plant- parasitic nematodes and Rhizoctonia solani in potato fields Pia Björsell Agricultural Programme – Soil and Plant Science Master’s thesis Uppsala 2015 Independent project/Degree project / SLU, Department of Ecology 2015:8 Interactions between some plant-parasitic nematodes and Rhizoctonia solani in potato fields Pia Björsell Supervisor: Maria Viketoft, SLU, Department of Ecology Assistant supervisor: Eva Edin, SLU, Department of Forest Mycology and Plant Pathology Examiner: Jonathan Yuen, SLU, Department of Forest Mycology and Plant Pathology Credits: 30 hec Level: A2E Course title: Independent Project/Degree Project in Biology – Master’s thesis – 30 hp Course code: EX0565 Programme/education: Agricultural Programme – Soil and Plant Science Place of publication: Uppsala Year of publication: 2015 Cover picture: Large picture: Potato field in Östergötland. Small picture top right: potato plant affected by R. solani. Small picture bottom left: nematodes under high magnification. Photos: Pia Björsell Title of series: Independent project/Degree project / SLU, Department of Ecology Part no: 2015:8 Online publication: http://stud.epsilon.slu.se Keywords: Rhizoctonia solani, Pratylenchus, Trichodoridae, Globodera, synergistic inter- actions, disease complex, Solanum tuberosum, spatial distribution, stem canker Sveriges lantbruksuniversitet Swedish University of Agricultural Sciences Faculty of Natural Resources and Agricultural Sciences Department of Ecology Abstract The pathogenic fungus Rhizoctonia solani causes major economic losses for potato producers in Sweden. The producers, as well as advisors, have reported possible increases in severity of R. solani when free-living plant parasitic nematodes are present and active. There are several examples in the literature of interactions be- tween pathogens where one, or both species, support or increase the damage made by the other organism. -

Potato Cyst Nematodes
Potato Cyst Nematodes Figure 1. Swollen females of the white potato cyst nematode (G.pallida) on potato roots (x10) Hosts Potato is the most important host for both Globodera pallida (the white potato cyst nematode) and G. rostochiensis (the yellow potato cyst nematode) (Fig.2). However, potato cyst nematodes (PCN) also attack other solanaceous plants, e.g. tomato, aubergine and, occasionally, weeds such as bittersweet (Solatium dulcamara). Each species of PCN has several pathotypes; these differ in their ability to multiply on different potato cultivars. Geographical Distribution Widespread in Europe, including the UK; also South America, where they originated. Present to a more limited extent in all other continents. The number and range of pathotypes vary from country to country. PLANT PEST FACTSHEET Sources Mainly introduced by cysts of the nematode in soil attached to potato tubers, farm machinery or footwear; they are only rarely introduced by infested tubers, if certified seed is used. Cysts are also spread locally by wind and floodwater. Description and Life Cycle The juveniles and adult male nematodes are worm-like, less than 1 mm long and transparent. They feed within the roots and are not easily seen. As each female matures it swells and becomes almost spherical, bursting through the root wall. Only Sand Hutton, York Tel +44 (0)1904 462 000 E-mail [email protected] YO41 1LZ, UK Fax +44 (0)1904 462 111 www.defra.gov.uk/fera the head of the female remains embedded in the root. The swollen female is shiny, spherical, less than 1 mm in diameter and initially white or cream-coloured.