Biochemical Basis for Differential Deoxyadenosine Toxicity To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Deoxyguanosine Cytotoxicity by a Novel Inhibitor of Furine Nucleoside Phosphorylase, 8-Amino-9-Benzylguanine1
[CANCER RESEARCH 46, 519-523, February 1986] Potentiation of 2'-Deoxyguanosine Cytotoxicity by a Novel Inhibitor of Furine Nucleoside Phosphorylase, 8-Amino-9-benzylguanine1 Donna S. Shewach,2 Ji-Wang Chern, Katherine E. Pillóte,Leroy B. Townsend, and Peter E. Daddona3 Departments of Internal Medicine [D.S.S., P.E.D.], Biological Chemistry [P.E.D.], and Medicinal Chemistry [J-W.C., K.E.P., L.B.T.], University ol Michigan, Ann Arbor, Michigan 48109 ABSTRACT to the ADA-deficient disease state (2). PNP is an essential enzyme of the purine salvage pathway, We have synthesized and evaluated a series of 9-substituted catalyzing the phosphorolysis of guanosine, inosine, and their analogues of 8-aminoguanine, a known inhibitor of human purine 2'-deoxyribonucleoside derivatives to the respective purine nucleoside phosphorylase (PNP) activity. The ability of these bases. To date, several inhibitors of PNP have been identified, agents to inhibit PNP has been investigated. All compounds were and most of these compounds resemble purine bases or nucleo found to act as competitive (with inosine) inhibitors of PNP, with sides. The most potent inhibitors exhibit apparent K¡values in K¡values ranging from 0.2 to 290 /¿M.Themost potent of these the range of 10~6to 10~7 M (9-12). Using partially purified human analogues, 8-amino-9-benzylguanine, exhibited a K, value that erythrocyte PNP, the diphosphate derivative of acyclovir dis was 4-fold lower than that determined for the parent base, 8- played K¡values of 5.1 x 10~7 to 8.7 x 10~9 M, depending on aminoguanine. -

Inhibitory Effects of Cordycepin on Platelet Activation Via Regulation of Cyclic Adenosine Monophosphate-Downstream Pathway
Biomedical Science Letters 2017, 23(3): 251~260 Original Article https://doi.org/10.15616/BSL.2017.23.3.251 eISSN : 2288-7415 Inhibitory Effects of Cordycepin on Platelet Activation via Regulation of Cyclic Adenosine Monophosphate-downstream Pathway Dong-Ha Lee† Department of Biomedical Laboratory Science, Korea Nazarene University, Cheonan 31172, Korea Platelet activation is essential at the sites of vascular injury, which leads to hemostasis through adhesion, aggregation, and secretion process. However, potent and continuous platelet activation may be an important reason of circulatory disorders. Therefore, proper regulation of platelet activation may be an effective treatment for vascular diseases. In this research, inhibitory effects of cordycepin (3'-deoxyadenosine) on platelet activation were determined. As the results, cordycepin increased cAMP and cGMP, which are intracellular Ca2+-antagonists. In addition, cordycepin reduced collagen- 2+ elevated [Ca ]i mobilization, which was increased by a cAMP-dependent protein kinase (PKA) inhibitor (Rp-8-Br- cAMPS), but not a cGMP-protein kinase (PKG) inhibitor (Rp-8-Br-cGMPS). Furthermore, cordycepin increased IP3RI 1756 2+ (Ser ) phosphorylation, indicating inhibition of IP3-mediated Ca release from internal store via the IP3RI, which was strongly inhibited by Rp-8-Br-cAMPS, but was not so much inhibited by Rp-8-Br-cGMPS. These results suggest that the 2+ 1756 reduction of [Ca ]i mobilization is caused by the cAMP/A-kinase-dependent IP3RI (Ser ) phosphorylation. In addition, cordycepin increased the phosphorylation of VASP (Ser157) known as PKA substrate, but not VASP (Ser239) known as PKG substrate. Cordycepin-induced VASP (Ser157) phosphorylation was inhibited by Rp-8-Br-cAMPS, but was not inhibited by Rp-8-Br-cGMPS, and cordycepin inhibited collagen-induced fibrinogen binding to αIIb/β3, which was increased by Rp-8-Br-cAMPS, but was not inhibited by Rp-8-Br-cGMPS. -

Leeds Thesis Template
Determining the Link Between Genome Integrity and Seed Quality Robbie Michael Gillett Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds Faculty of Biological Sciences School of Biology January, 2017 - ii - The candidate confirms that the work submitted is his own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Robbie M. Gillett to be identified as Author of this work has been asserted by him in accordance with the Copyright, Designs and Patents Act 1988. © 2016 The University of Leeds and Robbie Michael Gillett - iii - Acknowledgements I would like to thank my supervisor Dr Christopher West and co-supervisor Dr. Wanda Waterworth for all of their support, expertise and incredible patience and for always being available to help. I would like to thank Aaron Barrett, James Cooper, Valérie Tennant, and all my other friends at university for their help throughout my PhD. Thanks to Vince Agboh and Grace Hoysteed for their combined disjointedness and to Ashley Hines, Daniel Johnston and Darryl Ransom for being a source of entertainment for many years. I would like to thank my international Sona friends, Lindsay Hoffman and Jan Maarten ten Katen. Finally unreserved thanks to my loving parents who supported me throughout the tough times and to my Grandma and Granddad, Jean and Dennis McCarthy, who were always very vocal with their love, support and pride. -

The Microbiota-Produced N-Formyl Peptide Fmlf Promotes Obesity-Induced Glucose
Page 1 of 230 Diabetes Title: The microbiota-produced N-formyl peptide fMLF promotes obesity-induced glucose intolerance Joshua Wollam1, Matthew Riopel1, Yong-Jiang Xu1,2, Andrew M. F. Johnson1, Jachelle M. Ofrecio1, Wei Ying1, Dalila El Ouarrat1, Luisa S. Chan3, Andrew W. Han3, Nadir A. Mahmood3, Caitlin N. Ryan3, Yun Sok Lee1, Jeramie D. Watrous1,2, Mahendra D. Chordia4, Dongfeng Pan4, Mohit Jain1,2, Jerrold M. Olefsky1 * Affiliations: 1 Division of Endocrinology & Metabolism, Department of Medicine, University of California, San Diego, La Jolla, California, USA. 2 Department of Pharmacology, University of California, San Diego, La Jolla, California, USA. 3 Second Genome, Inc., South San Francisco, California, USA. 4 Department of Radiology and Medical Imaging, University of Virginia, Charlottesville, VA, USA. * Correspondence to: 858-534-2230, [email protected] Word Count: 4749 Figures: 6 Supplemental Figures: 11 Supplemental Tables: 5 1 Diabetes Publish Ahead of Print, published online April 22, 2019 Diabetes Page 2 of 230 ABSTRACT The composition of the gastrointestinal (GI) microbiota and associated metabolites changes dramatically with diet and the development of obesity. Although many correlations have been described, specific mechanistic links between these changes and glucose homeostasis remain to be defined. Here we show that blood and intestinal levels of the microbiota-produced N-formyl peptide, formyl-methionyl-leucyl-phenylalanine (fMLF), are elevated in high fat diet (HFD)- induced obese mice. Genetic or pharmacological inhibition of the N-formyl peptide receptor Fpr1 leads to increased insulin levels and improved glucose tolerance, dependent upon glucagon- like peptide-1 (GLP-1). Obese Fpr1-knockout (Fpr1-KO) mice also display an altered microbiome, exemplifying the dynamic relationship between host metabolism and microbiota. -

Plasma Deoxyadenosine, Adenosine, and Erythrocyte Deoxyatp Are Elevated at Birth in an Adenosine Deaminase-Deficient Child
Plasma deoxyadenosine, adenosine, and erythrocyte deoxyATP are elevated at birth in an adenosine deaminase-deficient child. R Hirschhorn, … , A Rubinstein, P Papageorgiou J Clin Invest. 1980;65(3):768-771. https://doi.org/10.1172/JCI109725. Research Article We have determined concentrations of adenosine, deoxyadenosine, and deoxyATP (dATP) in cord blood from an infant prenatally diagnosed as ADA deficient. Plasma deoxyadenosine and adenosine were already elevated in cord blood (0.7 and 0.5 microM vs. normal of less than 0.07 microM). Elevation of plasma deoxyadenosine has not previously been documented in these children. Erythrocyte dATP content was also elevated at birth (215 nmol/ml packed erythrocytes vs. normal of 2.9). These elevated concentrations of adenosine, deoxyadenosine, and dATP are similar to those we observed in another older adenosine deaminase-deficient patient and may explain the impaired immune function and lymphopenia seen at birth. Find the latest version: https://jci.me/109725/pdf RAPID PUBLICATIONS Plasma Deoiyadenosine, Adenosine, and Erythrocyte deoxyATP are Elevated at Birth in an Adenosine Deaminase-deficient Child ROCHELLE HIRSCHHORN and VIVIAN ROEGNER, Department of Medicine, New York University School of Medicine, New York 10016 ARYE RUBINSTEIN, Department of Pediatrics, Albert Einstein College of Medicine, New York 10461 PHOTINI PAPAGEORGIOU, Department of Pediatrics, Rutgers University Medical School, New Brunswick, Netv Jersey 08854 A B S T RA C T We have determined concentrations of amounts of deoxyadenosine, another substrate of ADA, adenosine, deoxyadenosine, and deoxyATP (dATP) in in their urine (4-11). Additionally, deoxyATP (dATP), a cord blood from an infant prenatally diagnosed as ADA metabolite of deoxyadenosine, is markedly increased deficient. -

Style Specifications Thesis
Structural Studies of Salvage Enzymes in Nucleotide Biosynthesis Martin Welin Faculty of Natural Resources and Agricultural Sciences Department of Molecular Biology Uppsala Doctoral thesis Swedish University of Agricultural Sciences Uppsala 2007 Acta Universitatis Agriculturae Sueciae 2007:21 ISSN 1652-6880 ISBN 978-91-576-7320-6 © 2007 Martin Welin, Uppsala Tryck: SLU Service/Repro, Uppsala 2007 Abstract Welin, M., 2007. Structural Studies of Salvage Enzymes in Nucleotide Biosynthesis. Doctoral dissertation. ISSN 1652-6880, ISBN 978-91-576-7320-6 There are two routes to produce deoxyribonucleoside triphosphates (dNTPs) precursors for DNA synthesis, the de novo and the salvage pathways. Deoxyribonucleoside kinases (dNKs) perform the initial phosphorylation of deoxyribonucleosides (dNs). Furthermore, they can act as activators for several medically important nucleoside analogs (NAs) for treatment against cancer or viral infections. Several disorders are characterized by mutations in enzymes involved in the nucleotide biosynthesis, such as Lesch-Nyhan disease that is linked to hypoxanthine guanine phosphoribosyltransferase (HPRT). In this thesis, the structures of human thymidine kinase 1 (TK1), a mycoplasmic deoxyadenosine kinase (Mm-dAK), and phosphoribosyltransferase domain containing 1 (PRTFDC1) are presented. Furthermore, a structural investigation of Drosophila melanogaster dNK (Dm-dNK) N64D mutant was carried out. The obtained structural information reveals the basis for substrate specificity for TKs and the bacterial dAKs. The TK1 revealed a structure different from other known dNK structures, containing an α/β domain similar to the RecA-F1ATPase family, and a lasso-like domain stabilized by a structural zinc. The Mm-dAK structure was similar to its human counterparts, but with some alterations in the proximity of the active site. -

One-Pot Multi-Enzymatic Production of Purine Derivatives with Application in Pharmaceutical and Food Industry
Article One-Pot Multi-Enzymatic Production of Purine Derivatives with Application in Pharmaceutical and Food Industry Javier Acosta 1, Jon del Arco 1, Sara Martinez-Pascual 1, Vicente Javier Clemente-Suárez 1,2 and Jesús Fernández-Lucas 1,2,* 1 Applied Biotechnology Group, European University of Madrid, c/ Tajo s/n, Villaviciosa de Odón, Madrid 28670, Spain; [email protected] (J.A.); [email protected] (J.d.A.); [email protected] (S.M.-P.); [email protected] (V.J.C.-S.) 2 Grupo de Investigación en Desarrollo Agroindustrial Sostenible, Universidad de la Costa, CUC, Calle 58 # 55-66, Barranquilla 080002, Colombia * Correspondence: [email protected]; Tel.: +34-912-115147 Received: 30 November 2017; Accepted: 28 December 2017; Published: 1 January 2018 Abstract: Biocatalysis reproduce nature’s synthetic strategies in order to synthesize different organic compounds. Natural metabolic pathways usually involve complex networks to support cellular growth and survival. In this regard, multi-enzymatic systems are valuable tools for the production of a wide variety of organic compounds. Methods: The production of different purine nucleosides and nucleoside-5′-monophosphates has been performed for first time, catalyzed by the sequential action of 2′-deoxyribosyltransferase from Lactobacillus delbrueckii (LdNDT) and hypoxanthine-guanine-xanthine phosphoribosyltransferase from Thermus themophilus HB8 (TtHGXPRT). Results: The biochemical characterization of LdNDT reveals that the enzyme is active and stable in a broad range of pH, temperature, and ionic strength. Substrate specificity studies showed a high promiscuity in the recognition of purine analogues. Finally, the enzymatic production of different purine derivatives was performed to evaluate the efficiency of multi-enzymatic system LdNDT/TtHGXPRT. -

The Metabolic Building Blocks of a Minimal Cell Supplementary
The metabolic building blocks of a minimal cell Mariana Reyes-Prieto, Rosario Gil, Mercè Llabrés, Pere Palmer and Andrés Moya Supplementary material. Table S1. List of enzymes and reactions modified from Gabaldon et. al. (2007). n.i.: non identified. E.C. Name Reaction Gil et. al. 2004 Glass et. al. 2006 number 2.7.1.69 phosphotransferase system glc + pep → g6p + pyr PTS MG041, 069, 429 5.3.1.9 glucose-6-phosphate isomerase g6p ↔ f6p PGI MG111 2.7.1.11 6-phosphofructokinase f6p + atp → fbp + adp PFK MG215 4.1.2.13 fructose-1,6-bisphosphate aldolase fbp ↔ gdp + dhp FBA MG023 5.3.1.1 triose-phosphate isomerase gdp ↔ dhp TPI MG431 glyceraldehyde-3-phosphate gdp + nad + p ↔ bpg + 1.2.1.12 GAP MG301 dehydrogenase nadh 2.7.2.3 phosphoglycerate kinase bpg + adp ↔ 3pg + atp PGK MG300 5.4.2.1 phosphoglycerate mutase 3pg ↔ 2pg GPM MG430 4.2.1.11 enolase 2pg ↔ pep ENO MG407 2.7.1.40 pyruvate kinase pep + adp → pyr + atp PYK MG216 1.1.1.27 lactate dehydrogenase pyr + nadh ↔ lac + nad LDH MG460 1.1.1.94 sn-glycerol-3-phosphate dehydrogenase dhp + nadh → g3p + nad GPS n.i. 2.3.1.15 sn-glycerol-3-phosphate acyltransferase g3p + pal → mag PLSb n.i. 2.3.1.51 1-acyl-sn-glycerol-3-phosphate mag + pal → dag PLSc MG212 acyltransferase 2.7.7.41 phosphatidate cytidyltransferase dag + ctp → cdp-dag + pp CDS MG437 cdp-dag + ser → pser + 2.7.8.8 phosphatidylserine synthase PSS n.i. cmp 4.1.1.65 phosphatidylserine decarboxylase pser → peta PSD n.i. -

Deoxyadenosine Triphosphate As a Mediator of Deoxyguanosine Toxicity in Cultured T Lymphoblasts
Deoxyadenosine triphosphate as a mediator of deoxyguanosine toxicity in cultured T lymphoblasts. G J Mann, R M Fox J Clin Invest. 1986;78(5):1261-1269. https://doi.org/10.1172/JCI112710. Research Article The mechanism by which 2'-deoxyguanosine is toxic for lymphoid cells is relevant both to the severe cellular immune defect of inherited purine nucleoside phosphorylase (PNP) deficiency and to attempts to exploit PNP inhibitors therapeutically. We have studied the cell cycle and biochemical effects of 2'-deoxyguanosine in human lymphoblasts using the PNP inhibitor 8-aminoguanosine. We show that cytostatic 2'-deoxyguanosine concentrations cause G1-phase arrest in PNP-inhibited T lymphoblasts, regardless of their hypoxanthine guanine phosphoribosyltransferase status. This effect is identical to that produced by 2'-deoxyadenosine in adenosine deaminase-inhibited T cells. 2'-Deoxyguanosine elevates both the 2'-deoxyguanosine-5'-triphosphate (dGTP) and 2'-deoxyadenosine-5'-triphosphate (dATP) pools; subsequently pyrimidine deoxyribonucleotide pools are depleted. The time course of these biochemical changes indicates that the onset of G1-phase arrest is related to increase of the dATP rather than the dGTP pool. When dGTP elevation is dissociated from dATP elevation by coincubation with 2'-deoxycytidine, dGTP does not by itself interrupt transit from the G1 to the S phase. It is proposed that dATP can mediate both 2'-deoxyguanosine and 2'-deoxyadenosine toxicity in T lymphoblasts. Find the latest version: https://jci.me/112710/pdf Deoxyadenosine Triphosphate as a Mediator of Deoxyguanosine Toxicity in Cultured T Lymphoblasts G. J. Mann and R. M. Fox Ludwig Institute for Cancer Research (Sydney Branch), University ofSydney, Sydney, New South Wales 2006, Australia Abstract urine of PNP-deficient individuals, with elevation of plasma inosine and guanosine and mild hypouricemia (3). -

Q 297 Suppl USE
The following supplement accompanies the article Atlantic salmon raised with diets low in long-chain polyunsaturated n-3 fatty acids in freshwater have a Mycoplasma dominated gut microbiota at sea Yang Jin, Inga Leena Angell, Simen Rød Sandve, Lars Gustav Snipen, Yngvar Olsen, Knut Rudi* *Corresponding author: [email protected] Aquaculture Environment Interactions 11: 31–39 (2019) Table S1. Composition of high- and low LC-PUFA diets. Stage Fresh water Sea water Feed type High LC-PUFA Low LC-PUFA Fish oil Initial fish weight (g) 0.2 0.4 1 5 15 30 50 0.2 0.4 1 5 15 30 50 80 200 Feed size (mm) 0.6 0.9 1.3 1.7 2.2 2.8 3.5 0.6 0.9 1.3 1.7 2.2 2.8 3.5 3.5 4.9 North Atlantic fishmeal (%) 41 40 40 40 40 30 30 41 40 40 40 40 30 30 35 25 Plant meals (%) 46 45 45 42 40 49 48 46 45 45 42 40 49 48 39 46 Additives (%) 3.3 3.2 3.2 3.5 3.3 3.4 3.9 3.3 3.2 3.2 3.5 3.3 3.4 3.9 2.6 3.3 North Atlantic fish oil (%) 9.9 12 12 15 16 17 18 0 0 0 0 0 1.2 1.2 23 26 Linseed oil (%) 0 0 0 0 0 0 0 6.8 8.1 8.1 9.7 11 10 11 0 0 Palm oil (%) 0 0 0 0 0 0 0 3.2 3.8 3.8 5.4 5.9 5.8 5.9 0 0 Protein (%) 56 55 55 51 49 47 47 56 55 55 51 49 47 47 44 41 Fat (%) 16 18 18 21 22 22 22 16 18 18 21 22 22 22 28 31 EPA+DHA (% diet) 2.2 2.4 2.4 2.9 3.1 3.1 3.1 0.7 0.7 0.7 0.7 0.7 0.7 0.7 4 4.2 Table S2. -
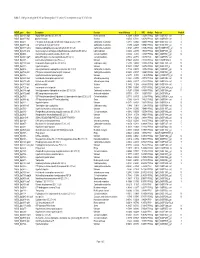
Table 7. GAS Genes Upregulated (+Z) and Downregulated (-Z) on Day 32 in Comparison to Day 16 of Infection
Table 7. GAS genes upregulated (+Z) and downregulated (-Z) on day 32 in comparison to day 16 of infection. M5005_gene Gene Description Function mean difference Z NFD Analysis Probe-set Probe # M5005_Spy1808 argS Arginyl-tRNA synthetase (EC 6.1.1.19) Protein synthesis -1.72298 -4.95958 0.14279 PPA1&2 SpM1_ChORF2151_s_at 8 M5005_Spy0317 hlyX putative hemolysin Virulence -2.02534 -4.71231 0.30786 PPA1&2 SpM1_ChORF0378_s_at 5 M5005_Spy0475 - PTS system, beta-glucoside-specific IIABC component (EC 2.7.1.69) Carbohydrate metabolism -1.47813 -4.48425 0.62109 PPA1&2 SpM1_ChORF0572_s_at 4 M5005_Spy0577 atpF ATP synthase B chain (EC 3.6.3.14) Carbohydrate metabolism -1.7496 -4.45203 0.68541 PPA1&2 SpM1_ChORF0756_s_at 11 M5005_Spy1637 lacB.2 Galactose-6-phosphate isomerase lacB subunit (EC 5.3.1.26) Carbohydrate metabolism -1.94768 -4.37397 0.86962 PPA1&2 SpM18_ChORF1990_s_at 15 M5005_Spy1485 accA Acetyl-coenzyme A carboxylase carboxyl transferase subunit beta (EC 6.4.1.2) Lipid metabolism -1.67854 -4.23953 1.30699 PPA1&2 SpM1_ChORF1743_s_at 4 M5005_Spy0500 - N-acetylmuramoyl-L-alanine amidase (EC 3.5.1.28) Cell wall metabolism -1.54852 -4.2346 1.32657 PPA1&2 SpM1_ChORF0601_s_at 12 M5005_Spy0608 rgpFc alpha-L-Rha alpha-1,3-L-rhamnosyltransferase (EC 2.4.1.-) Cell wall metabolism -0.77914 -4.07772 0.58709 PPA3 SpM1_ChORF0792_s_at M5005_Spy1697 - 4-amino-4-deoxychorismate lyase (EC 4.-.-.-) Unknown -1.55045 -4.04336 2.35339 PPA1&2 SpM18_ChORF2056_at 12 M5005_Spy1108 metK S-adenosylmethionine synthetase (EC 2.5.1.6) Cellular processing -1.57308 -3.94036 -

Increased Excretion of Modified Adenine Nucleosides by Children with Adenosine Dearninase Deficiency
Pediatr. Res. 16: 362-369 (1982) Increased Excretion of Modified Adenine Nucleosides by Children with Adenosine Dearninase Deficiency ROCHELLE HIRSCHHORN,'"~ HOWARD RATECH, ARYE RUBINSTEIN, PHOTINI PAPAGEORGIOU, HERNANT KESARWALA, ERWIN GELFAND, AND VIVIEN ROEGNER-MANISCALCO Departments of Medicine and Pathology, New York University School of Medicine, New York, New York [R.H., H.R., and V.R.-M.];Department of Pediatrics, Albert Einstein College of Medicine, Bronx, New York [A.R.]; Department of Pediatrics, Rutgers University Medical ~chool,Piscataway, New Jersey [P.P., and H.K.]; and Department of Pediatrics, Hospital for Sick Children, Toronto, Ontario, Canada [E. G.] Summary tially in, and prevents proliferation of, irnmunocompetent cells, primarily of the T cell class (2, 5, 6, 23, 38, 49, 54). There is also We have identified seven adenine nucleosides in urines of un- in vivo and/or in vitro evidence for alternative mechanisms of treated adenosine deaminase (ADA) deficient patients, four of toxicity, which would operate via depletion of pyrimidine pools, which (adenosine, 2'-deoxyadenosine, 1-methyladenosine and N6- depletion of phosphoribosyl pyrophosphate and increases in cyclic methyladenosine) have been previously identified in urines of AMP or S-adenosyl homocysteine (16, 21, 24, 40, 46, 55). All of normals and/or ADA deficient patients. We confirm that ADA these mechanisms are dependent on accumulation of the substrates deficient patients excrete markedly increased amounts of 2'-deox- of ADA, adenosine and 2'-deoxyadenosine. yadenosine (582 k 363 versus normal of < 0.1 nmoles/mg creati- In addition to adenosine and 2'-deoxyadenosine, several other nine) and increased amounts of adenosine (29.4 & 5.7 versus modified adenine nucleosides occur naturally (17, 19) and are normal of 4.12 & 1.0 nmoles/mg creatinine).