Basic Concepts of Thermodynamics Every Science Has Its Own Unique Vocabulary Associated with It
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
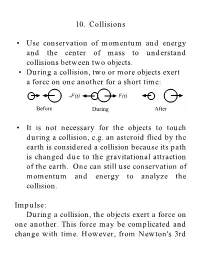
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Thermodynamics Notes
Thermodynamics Notes Steven K. Krueger Department of Atmospheric Sciences, University of Utah August 2020 Contents 1 Introduction 1 1.1 What is thermodynamics? . .1 1.2 The atmosphere . .1 2 The Equation of State 1 2.1 State variables . .1 2.2 Charles' Law and absolute temperature . .2 2.3 Boyle's Law . .3 2.4 Equation of state of an ideal gas . .3 2.5 Mixtures of gases . .4 2.6 Ideal gas law: molecular viewpoint . .6 3 Conservation of Energy 8 3.1 Conservation of energy in mechanics . .8 3.2 Conservation of energy: A system of point masses . .8 3.3 Kinetic energy exchange in molecular collisions . .9 3.4 Working and Heating . .9 4 The Principles of Thermodynamics 11 4.1 Conservation of energy and the first law of thermodynamics . 11 4.1.1 Conservation of energy . 11 4.1.2 The first law of thermodynamics . 11 4.1.3 Work . 12 4.1.4 Energy transferred by heating . 13 4.2 Quantity of energy transferred by heating . 14 4.3 The first law of thermodynamics for an ideal gas . 15 4.4 Applications of the first law . 16 4.4.1 Isothermal process . 16 4.4.2 Isobaric process . 17 4.4.3 Isosteric process . 18 4.5 Adiabatic processes . 18 5 The Thermodynamics of Water Vapor and Moist Air 21 5.1 Thermal properties of water substance . 21 5.2 Equation of state of moist air . 21 5.3 Mixing ratio . 22 5.4 Moisture variables . 22 5.5 Changes of phase and latent heats . -

Thermodynamics of Solar Energy Conversion in to Work
Sri Lanka Journal of Physics, Vol. 9 (2008) 47-60 Institute of Physics - Sri Lanka Research Article Thermodynamic investigation of solar energy conversion into work W.T.T. Dayanga and K.A.I.L.W. Gamalath Department of Physics, University of Colombo, Colombo 03, Sri Lanka Abstract Using a simple thermodynamic upper bound efficiency model for the conversion of solar energy into work, the best material for a converter was obtained. Modifying the existing detailed terrestrial application model of direct solar radiation to include an atmospheric transmission coefficient with cloud factors and a maximum concentration ratio, the best shape for a solar concentrator was derived. Using a Carnot engine in detailed space application model, the best shape for the mirror of a concentrator was obtained. A new conversion model was introduced for a solar chimney power plant to obtain the efficiency of the power plant and power output. 1. INTRODUCTION A system that collects and converts solar energy in to mechanical or electrical power is important from various aspects. There are two major types of solar power systems at present, one using photovoltaic cells for direct conversion of solar radiation energy in to electrical energy in combination with electrochemical storage and the other based on thermodynamic cycles. The efficiency of a solar thermal power plant is significantly higher [1] compared to the maximum efficiency of twenty percent of a solar cell. Although the initial cost of a solar thermal power plant is very high, the running cost is lower compared to the other power plants. Therefore most countries tend to build solar thermal power plants. -

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -
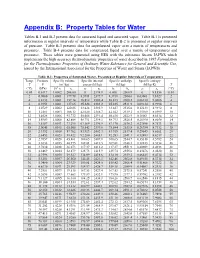
Appendix B: Property Tables for Water
Appendix B: Property Tables for Water Tables B-1 and B-2 present data for saturated liquid and saturated vapor. Table B-1 is presented information at regular intervals of temperature while Table B-2 is presented at regular intervals of pressure. Table B-3 presents data for superheated vapor over a matrix of temperatures and pressures. Table B-4 presents data for compressed liquid over a matrix of temperatures and pressures. These tables were generated using EES with the substance Steam_IAPWS which implements the high accuracy thermodynamic properties of water described in 1995 Formulation for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use, issued by the International Associated for the Properties of Water and Steam (IAPWS). Table B-1: Properties of Saturated Water, Presented at Regular Intervals of Temperature Temp. Pressure Specific volume Specific internal Specific enthalpy Specific entropy T P (m3/kg) energy (kJ/kg) (kJ/kg) (kJ/kg-K) T 3 (°C) (kPa) 10 vf vg uf ug hf hg sf sg (°C) 0.01 0.6117 1.0002 206.00 0 2374.9 0.000 2500.9 0 9.1556 0.01 2 0.7060 1.0001 179.78 8.3911 2377.7 8.3918 2504.6 0.03061 9.1027 2 4 0.8135 1.0001 157.14 16.812 2380.4 16.813 2508.2 0.06110 9.0506 4 6 0.9353 1.0001 137.65 25.224 2383.2 25.225 2511.9 0.09134 8.9994 6 8 1.0729 1.0002 120.85 33.626 2385.9 33.627 2515.6 0.12133 8.9492 8 10 1.2281 1.0003 106.32 42.020 2388.7 42.022 2519.2 0.15109 8.8999 10 12 1.4028 1.0006 93.732 50.408 2391.4 50.410 2522.9 0.18061 8.8514 12 14 1.5989 1.0008 82.804 58.791 2394.1 58.793 2526.5 -

HEAT and TEMPERATURE Heat Is a Type of ENERGY. When Absorbed
HEAT AND TEMPERATURE Heat is a type of ENERGY. When absorbed by a substance, heat causes inter-particle bonds to weaken and break which leads to a change of state (solid to liquid for example). Heat causing a phase change is NOT sufficient to cause an increase in temperature. Heat also causes an increase of kinetic energy (motion, friction) of the particles in a substance. This WILL cause an increase in TEMPERATURE. Temperature is NOT energy, only a measure of KINETIC ENERGY The reason why there is no change in temperature at a phase change is because the substance is using the heat only to change the way the particles interact (“stick together”). There is no increase in the particle motion and hence no rise in temperature. THERMAL ENERGY is one type of INTERNAL ENERGY possessed by an object. It is the KINETIC ENERGY component of the object’s internal energy. When thermal energy is transferred from a hot to a cold body, the term HEAT is used to describe the transferred energy. The hot body will decrease in temperature and hence in thermal energy. The cold body will increase in temperature and hence in thermal energy. Temperature Scales: The K scale is the absolute temperature scale. The lowest K temperature, 0 K, is absolute zero, the temperature at which an object possesses no thermal energy. The Celsius scale is based upon the melting point and boiling point of water at 1 atm pressure (0, 100o C) K = oC + 273.13 UNITS OF HEAT ENERGY The unit of heat energy we will use in this lesson is called the JOULE (J). -

Solar Thermal Energy
22 Solar Thermal Energy Solar thermal energy is an application of solar energy that is very different from photovol- taics. In contrast to photovoltaics, where we used electrodynamics and solid state physics for explaining the underlying principles, solar thermal energy is mainly based on the laws of thermodynamics. In this chapter we give a brief introduction to that field. After intro- ducing some basics in Section 22.1, we will discuss Solar Thermal Heating in Section 22.2 and Concentrated Solar (electric) Power (CSP) in Section 22.3. 22.1 Solar thermal basics We start this section with the definition of heat, which sometimes also is called thermal energy . The molecules of a body with a temperature different from 0 K exhibit a disordered movement. The kinetic energy of this movement is called heat. The average of this kinetic energy is related linearly to the temperature of the body. 1 Usually, we denote heat with the symbol Q. As it is a form of energy, its unit is Joule (J). If two bodies with different temperatures are brought together, heat will flow from the hotter to the cooler body and as a result the cooler body will be heated. Dependent on its physical properties and temperature, this heat can be absorbed in the cooler body in two forms, sensible heat and latent heat. Sensible heat is that form of heat that results in changes in temperature. It is given as − Q = mC p(T2 T1), (22.1) where Q is the amount of heat that is absorbed by the body, m is its mass, Cp is its heat − capacity and (T2 T1) is the temperature difference. -

The First Law of Thermodynamics for Closed Systems A) the Energy
Chapter 3: The First Law of Thermodynamics for Closed Systems a) The Energy Equation for Closed Systems We consider the First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle. Thus energy is transferred between the system and the surroundings in the form of heat and work, resulting in a change of internal energy of the system. Internal energy change can be considered as a measure of molecular activity associated with change of phase or temperature of the system and the energy equation is represented as follows: Heat (Q) Energy transferred across the boundary of a system in the form of heat always results from a difference in temperature between the system and its immediate surroundings. We will not consider the mode of heat transfer, whether by conduction, convection or radiation, thus the quantity of heat transferred during any process will either be specified or evaluated as the unknown of the energy equation. By convention, positive heat is that transferred from the surroundings to the system, resulting in an increase in internal energy of the system Work (W) In this course we consider three modes of work transfer across the boundary of a system, as shown in the following diagram: Source URL: http://www.ohio.edu/mechanical/thermo/Intro/Chapt.1_6/Chapter3a.html Saylor URL: http://www.saylor.org/me103#4.1 Attributed to: Israel Urieli www.saylor.org Page 1 of 7 In this course we are primarily concerned with Boundary Work due to compression or expansion of a system in a piston-cylinder device as shown above. -

Linear-Nonequilibrium Thermodynamics Theory for Coupled Heat and Mass Transport
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Chemical and Biomolecular Research Papers -- Yasar Demirel Publications Faculty Authors Series 2001 Linear-nonequilibrium thermodynamics theory for coupled heat and mass transport Yasar Demirel University of Nebraska - Lincoln, [email protected] Stanley I. Sandler University of Delaware, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/cbmedemirel Part of the Chemical Engineering Commons Demirel, Yasar and Sandler, Stanley I., "Linear-nonequilibrium thermodynamics theory for coupled heat and mass transport" (2001). Yasar Demirel Publications. 8. https://digitalcommons.unl.edu/cbmedemirel/8 This Article is brought to you for free and open access by the Chemical and Biomolecular Research Papers -- Faculty Authors Series at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Yasar Demirel Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Published in International Journal of Heat and Mass Transfer 44 (2001), pp. 2439–2451 Copyright © 2001 Elsevier Science Ltd. Used by permission. www.elsevier.comllocate/ijhmt Submitted November 5, 1999; revised September 6, 2000. Linear-nonequilibrium thermodynamics theory for coupled heat and mass transport Yasar Demirel and S. I. Sandler Center for Molecular and Engineering Thermodynamics, Department of Chemical Engineering, University of Delaware, Newark, DE 19716, USA Corresponding author — Y. Demirel Abstract Linear-nonequilibrium thermodynamics (LNET) has been used to express the entropy generation and dis- sipation functions representing the true forces and flows for heat and mass transport in a multicomponent fluid. These forces and flows are introduced into the phenomenological equations to formulate the cou- pling phenomenon between heat and mass flows. -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

Work and Energy Summary Sheet Chapter 6
Work and Energy Summary Sheet Chapter 6 Work: work is done when a force is applied to a mass through a displacement or W=Fd. The force and the displacement must be parallel to one another in order for work to be done. F (N) W =(Fcosθ)d F If the force is not parallel to The area of a force vs. the displacement, then the displacement graph + W component of the force that represents the work θ d (m) is parallel must be found. done by the varying - W d force. Signs and Units for Work Work is a scalar but it can be positive or negative. Units of Work F d W = + (Ex: pitcher throwing ball) 1 N•m = 1 J (Joule) F d W = - (Ex. catcher catching ball) Note: N = kg m/s2 • Work – Energy Principle Hooke’s Law x The work done on an object is equal to its change F = kx in kinetic energy. F F is the applied force. 2 2 x W = ΔEk = ½ mvf – ½ mvi x is the change in length. k is the spring constant. F Energy Defined Units Energy is the ability to do work. Same as work: 1 N•m = 1 J (Joule) Kinetic Energy Potential Energy Potential energy is stored energy due to a system’s shape, position, or Kinetic energy is the energy of state. motion. If a mass has velocity, Gravitational PE Elastic (Spring) PE then it has KE 2 Mass with height Stretch/compress elastic material Ek = ½ mv 2 EG = mgh EE = ½ kx To measure the change in KE Change in E use: G Change in ES 2 2 2 2 ΔEk = ½ mvf – ½ mvi ΔEG = mghf – mghi ΔEE = ½ kxf – ½ kxi Conservation of Energy “The total energy is neither increased nor decreased in any process. -

Heat Energy a Science A–Z Physical Series Word Count: 1,324 Heat Energy
Heat Energy A Science A–Z Physical Series Word Count: 1,324 Heat Energy Written by Felicia Brown Visit www.sciencea-z.com www.sciencea-z.com KEY ELEMENTS USED IN THIS BOOK The Big Idea: One of the most important types of energy on Earth is heat energy. A great deal of heat energy comes from the Sun’s light Heat Energy hitting Earth. Other sources include geothermal energy, friction, and even living things. Heat energy is the driving force behind everything we do. This energy gives us the ability to run, dance, sing, and play. We also use heat energy to warm our homes, cook our food, power our vehicles, and create electricity. Key words: cold, conduction, conductor, convection, energy, evaporate, fire, friction, fuel, gas, geothermal heat, geyser, heat energy, hot, insulation, insulator, lightning, liquid, matter, particles, radiate, radiant energy, solid, Sun, temperature, thermometer, transfer, volcano Key comprehension skill: Cause and effect Other suitable comprehension skills: Compare and contrast; classify information; main idea and details; identify facts; elements of a genre; interpret graphs, charts, and diagram Key reading strategy: Connect to prior knowledge Other suitable reading strategies: Ask and answer questions; summarize; visualize; using a table of contents and headings; using a glossary and bold terms Photo Credits: Front cover: © iStockphoto.com/Julien Grondin; back cover, page 5: © iStockphoto.com/ Arpad Benedek; title page, page 20 (top): © iStockphoto.com/Anna Ziska; pages 3, 9, 20 (bottom): © Jupiterimages Corporation;