3 Functional Structure of the Peritoneum As a Dialysing Membrane
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -
The Subperitoneal Space and Peritoneal Cavity: Basic Concepts Harpreet K
ª The Author(s) 2015. This article is published with Abdom Imaging (2015) 40:2710–2722 Abdominal open access at Springerlink.com DOI: 10.1007/s00261-015-0429-5 Published online: 26 May 2015 Imaging The subperitoneal space and peritoneal cavity: basic concepts Harpreet K. Pannu,1 Michael Oliphant2 1Department of Radiology, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA 2Department of Radiology, Wake Forest University School of Medicine, Winston-Salem, NC, USA Abstract The peritoneum is analogous to the pleura which has a visceral layer covering lung and a parietal layer lining the The subperitoneal space and peritoneal cavity are two thoracic cavity. Similar to the pleural cavity, the peri- mutually exclusive spaces that are separated by the toneal cavity is visualized on imaging if it is abnormally peritoneum. Each is a single continuous space with in- distended by fluid, gas, or masses. terconnected regions. Disease can spread either within the subperitoneal space or within the peritoneal cavity to Location of the abdominal and pelvic organs distant sites in the abdomen and pelvis via these inter- connecting pathways. Disease can also cross the peri- There are two spaces in the abdomen and pelvis, the toneum to spread from the subperitoneal space to the peritoneal cavity (a potential space) and the subperi- peritoneal cavity or vice versa. toneal space, and these are separated by the peritoneum (Fig. 1). Regardless of the complexity of development in Key words: Subperitoneal space—Peritoneal the embryo, the subperitoneal space and the peritoneal cavity—Anatomy cavity remain separated from each other, and each re- mains a single continuous space (Figs. -

Carcinomatous Cirrhosis of the Liver with Sarcomatosis of the Peritoneum 1
CARCINOMATOUS CIRRHOSIS OF THE LIVER WITH SARCOMATOSIS OF THE PERITONEUM 1 S. SANES, M.D., AND K. TERPLAN, M.D. (From tile Pathological Laboratory of the Buffalo General Hospital and School of Medicine, University of Buffalo) The following case is reported because of the occurrence of two different types of malignant neoplasm with typical portal cirrhosis of the liver. That a pathogenetic relationship exists between Laennec's cirrhosis and primary carcinoma of the liver is generally recognized. Whether the association of a peritoneal sarcoma with the cirrhosis in this case was more than a coincidence seemed an interesting point for discussion. REPORT OF CASE E. G., 11 white Italian male fifty-seven years old, was admitted to the Buffalo General Hospital on the service of Drs. N. G. Russell and A. H. Aaron, Nov. 25, 1934. He died Nov. 29, 1934. All his adult life he had partaken of large amounts of wine and whiskey daily. At the age of seventeen years he had suffered an attack of jaundice of several weeks' duration. The patient first began to lose weight and strength in 1932 and noticed that his skin was becoming dark. In March 1934 he complained of cramp-like abdominal pain, diarrhea, and bloating. The stools were watery. There was no nausea or vomiting. Upon hos pitalization, April 9, 1934, physical examination revealed that the pupils reacted to light and accommodation. The chest was emphysematous; breath sounds were diminished in both bases. The heart was regular; a systolic murmur was heard. The blood pressure was 118/70. The liver and spleen were palpable three finger breadths below the costal margin. -

7) Anatomy of OMENTUM
OMENTUM ANATOMY DEPARTMENT DR.SANAA AL-SHAARAWY Dr. Essam Eldin Salama OBJECTIVES • At the end of the lecture the students must know: • Brief knowledge about peritoneum as a thin serous membrane and its main parts; parietal and visceral. • The peritonial cavity and its parts the greater sac and the lesser sac (Omental bursa). • The peritoneal folds : omenta, mesenteries, and ligaments. • The omentum, as one of the peritonial folds • The greater omentum, its boundaries, and contents. • The lesser omentum, its boundaries, and contents. • The omental bursa, its boundaries. • The Epiploic foramen, its boundaries. • Mesentery of the small intestine, and ligaments of the liver. • Nerve supply of the peritoneum. • Clinical points. The peritoneum vIs a thin serous membrane, §Lining the wall of the abdominal and pelvic cavities, (the parietal peritoneum). §Covering the existing organs, (the visceral peritoneum). §The potential space between the two layers is the peritoneal cavity. Parietal Visceral The peritoneal Cavity vThe peritoneal cavity is the largest one in the body. vDivisions of the peritoneal cavity : §Greater sac; extends from Lesser Sac diaphragm down to the pelvis. §Lesser sac; lies behind the stomach. §Both cavities are interconnected through the epiploic foramen. §In male : the peritoneum is a closed sac . §In female : the sac is not completely closed because it Greater Sac communicates with the exterior through the uterine tubes, uterus and vagina. The peritoneum qIntraperitoneal and Intraperitoneal viscera retroperitoneal organs; describe the relationship between various organs and their peritoneal covering; §Intraperitonial structure; which is nearly totally covered by visceral peritoneum. §Retroperitonial structure; lies behind the peritoneum, and partially covered by visceral peritoneum. -

BODY CAVITIES and MESENTERY
73: BODY CAVITIES and MESENTERY We've already mentioned that all the organs in the body are wrapped in "bags" made of thin layers of connective tissue. These bags are often inside of other bags, or even inside of several bags. The largest bags define areas that we call body cavities. There are three main cavities: the thoracic cavity, the abdominal cavity and the pelvic cavity. The thoracic cavity is subdivided into three smaller cavities: the pleural cavity (containing the lungs), the mediastinum(in the middle), and the pericardial cavity (containing the heart). The pleural cavity is easy to understand because it simply contains the lungs. The pericardial cavity contains not only the heart itself, but the large blood vessels that come out of it, such as the aorta. The pericardial cavity is inside of the third cavity, the mediastinum. ("Media" means "middle" and "stinum" can refer to the "sternum," which is the bone that runs down the center of the ribcage.) The mediastinum contains not only the pericardial cavity but also part of the esophagus and trachea, the thymus (remember this organ from module 2 on the immune system?), and quite a few nerves and lymph nodes. The thin layers of connective tissues that surround these cavities are made primarily of collagen and elastin (produced by fibroblast cells) but they also contain some very tiny nerves and blood vessels, as well as cells that make serous fluid. As we've seen in the past few lessons, the diaphragm separates the thoracic cavity from the abdominal cavity. The abdominal cavity contains the stomach, the spleen, the tail of the pancreas, the last half of the duodenum, the small intestines, most of the large intestines, and the mesentery (thin layers of connective tissue that anchor the intestines to the back wall of the abdominal cavity). -
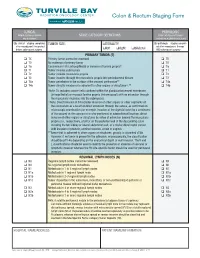
Colon & Rectum Staging Form
Colon & Rectum Staging Form CLINICAL PATHOLOGIC Extent of disease before STAGE CATEGORY DEFINITIONS Extent of disease through any treatment completion of definitive surgery y clinical – staging completed TUMOR SIZE: LATERALITY: y pathologic – staging complet- after neoadjuvant therapy but ed after neoadjuvant therapy before subsequent surgery left right bilateral AND subsequent surgery PRIMARY TUMOR (T) TX Primary tumor cannot be assessed TX T0 No evidence of primary tumor T0 Tis Carcinoma in situ: intraepithelial or invasion of lamina propria* Tis T1 Tumor invades submucosa T1 T2 Tumor invades muscularis propria T2 T3 Tumor invades through the muscularis propria into pericolorectal tissues T3 T4a Tumor penetrates to the surface of the visceral peritoneum** T4a T4b Tumor directly invades or is adherent to other organs or structures^,** T4b *Note: Tis includes cancer cells confined within the glandular basement membrane (intraepithelial) or mucosal lamina propria (intramucosal) with no extension through the muscularis mucosae into the submucosa. ^Note: Direct invasion in T4 includes invasion of other organs or other segments of the colorectum as a result of direct extension through the serosa, as confirmed on microscopic examination (for example, invasion of the sigmoid colon by a carcinoma of the cecum) or, for cancers in a retro-peritoneal or subperitoneal location, direct invasion of other organs or structures by virtue of extension beyond the muscularis propria (i.e., respectively, a tumor on the posterior wall of the descending colon invading the left kidney or lateral abdominal wall; or a mid or distal rectal cancer with invasion of prostate, seminal vesicles, cervix or vagina). **Tumor that is adherent to other organs or structures, grossly, is classified cT4b. -

The Formation of Peritoneal Adhesions
THE FORMATION OF PERITONEAL ADHESIONS Christian DellaCorte, Ph.D., C.M.T. The increased incidence of postoperative adhesions and their complications has focused attention on trying to understand the adhesion, adhesion formation, clinical consequences, and prevention of adhesion formation. Adhesions are highly differentiated, formed through an intricate process involving a complex organ, the peritoneum, whose surface lining is the key site in adhesion formation. The peritoneum, a serous membrane, serves a protective function for the contents of the abdominal cavity. Homeostasis is maintained by allowing exchange of molecules and production of peritoneal fluid. This provides an environment for optimal function of intra-abdominal organs. Forms of trauma to the peritoneum (i.e., mechanical, thermal, chemical, infectious, surgical, and/or ischemic) can result in the formation of peritoneal adhesions. In 1919, it was shown that peritoneal healing differed from that of skin. When the peritoneal membrane is traumatized, a dynamic response results that produces a series of steps toward rapid regeneration in approximately five to seven days of the injured peritoneum via re-epithelialization, irrespective of the size of injury. Microscopic studies showed the new peritoneal cells are derived from mesodermal cells of the underlying granulation tissue, multipotent mesenchymal cells that are able to take the form of fibroblasts or mesothelial cells. When a defect is made in the parietal peritoneum the entire surface becomes simultaneously epithelialized, differing from the gradual epidermalization from the borders as is found in skin wounds. Multiplication and migration of mesothelial cells from the margins of the wound may play a small part in the regenerative process, but it does not play a major role. -

The Mesocolon a Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization
ORIGINAL ARTICLE The Mesocolon A Histological and Electron Microscopic Characterization of the Mesenteric Attachment of the Colon Prior to and After Surgical Mobilization Kevin Culligan, MRCS,∗ Stewart Walsh, FRCSEd,∗ Colum Dunne, PhD,∗ Michael Walsh, PhD,† Siobhan Ryan, MB,‡ Fabio Quondamatteo, MD,‡ Peter Dockery, PhD,§ and J. Calvin Coffey, FRCSI∗¶ uring fetal development, the dorsal mesentery suspends the en- Background: Colonic mobilization requires separation of mesocolon from tire gastrointestinal tract from the posterior abdominal wall. The underlying fascia. Despite the surgical importance of planes formed by these D mesocolon is the adult remnant of that part of the dorsal mesentery structures, no study has formally characterized their microscopic features. associated with the colon.1 In the adult human, the transverse and The aim of this study was to determine the histological and electron micro- lateral sigmoid portions of the mesocolon are mobile whereas the as- scopic appearance of mesocolon, fascia, and retroperitoneum, prior to and cending, descending, and medial sigmoid portions are nonmobile and after colonic mobilization. attached to underlying retroperitoneum.2–4 Classic anatomic teaching Methods: In 24 cadavers, samples were taken from right, transverse, de- maintains that the ascending and descending mesocolon “disappear” scending, and sigmoid mesocolon. In 12 cadavers, specimens were stained during embryogenesis.5,6 In keeping with this, the identification of a with hematoxylin and eosin (3 sections) or Masson trichrome (3 sections). In right or left mesocolon in the adult is frequently depicted as anoma- the second 12 cadavers, lymphatic channels were identified by staining im- lous rather than accepted as an anatomic norm.7 Accordingly, the munohistochemically for podoplanin. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

ABDOMINOPELVIC CAVITY and PERITONEUM Dr
ABDOMINOPELVIC CAVITY AND PERITONEUM Dr. Milton M. Sholley SUGGESTED READING: Essential Clinical Anatomy 3 rd ed. (ECA): pp. 118 and 135141 Grant's Atlas Figures listed at the end of this syllabus. OBJECTIVES:Today's lectures are designed to explain the orientation of the abdominopelvic viscera, the peritoneal cavity, and the mesenteries. LECTURE OUTLINE PART 1 I. The abdominopelvic cavity contains the organs of the digestive system, except for the oral cavity, salivary glands, pharynx, and thoracic portion of the esophagus. It also contains major systemic blood vessels (aorta and inferior vena cava), parts of the urinary system, and parts of the reproductive system. A. The space within the abdominopelvic cavity is divided into two contiguous portions: 1. Abdominal portion that portion between the thoracic diaphragm and the pelvic brim a. The lower part of the abdominal portion is also known as the false pelvis, which is the part of the pelvis between the two iliac wings and above the pelvic brim. Sagittal section drawing Frontal section drawing 2. Pelvic portion that portion between the pelvic brim and the pelvic diaphragm a. The pelvic portion of the abdominopelvic cavity is also known as the true pelvis. B. Walls of the abdominopelvic cavity include: 1. The thoracic diaphragm (or just “diaphragm”) located superiorly and posterosuperiorly (recall the domeshape of the diaphragm) 2. The lower ribs located anterolaterally and posterolaterally 3. The posterior abdominal wall located posteriorly below the ribs and above the false pelvis and formed by the lumbar vertebrae along the posterior midline and by the quadratus lumborum and psoas major muscles on either side 4. -

Primary Retroperitoneal Mucinous Cystadenoma
Case Reports Primary retroperitoneal mucinous cystadenoma Malak S. Abedalthagafi, MD, Patrick G. Jackson, MD, Metin Ozdemirli, MD, PhD. rimary mucinous cystadenomas of the ABSTRACT Pretroperitoneum are extremely rare tumors. Although very rare cases were reported in men and children, these tumors are found exclusively in تتضمن أورام خلف الصفاق اﻷولي: السرطان الكيسي املخاطي، women.1-3 Like most retroperitoneal tumors, they can اﻷورام املخاطية ذات احلد الفاصل، اﻷورام النادرة واملتواجدة في cause symptoms through exertion of pressure or by النساء واملتضمنة احلزام املخاطي. وحيث أن خلف الصفاق اﻷولي .obstructing adjacent organs if they are large enough ﻻ يحتوي على ظاهرة مخاطية، تبقى نظرية حدوث هذه اﻷورام .They have potential for malignant transformation غير معروفة. نستنتج أن حدوث هذه اﻷورام قد يأتي من اﻷورام There is no unanimous opinion on the genesis of املسخية، أو من املبايض الزائدة، أو من التحول املخاطي للطبقة these tumors and due to their extreme rarity, their املتوسطة خللف الصفاق. نستعرض في هذا التقرير حالة للخدام histogenesis, biological behavior, and their optimal املخاطي خلف الصفاق اﻷولي ملريضة تبلغ من العمر 44 ًعاما، والتي ,management remains at a speculative level. In this paper حضرت بسبب ورم بطني. بعد استئصال الورم بجراحة املنظار we present a case of primary retroperitoneal mucinous لم يكن هناك أية أثر لعودة الورم بعد 16 شهرا.ً الشكل املجهري cystadenoma and review the clinicopathological features, therapeutic options, and outcome in respect والتحليل للصبغات النسيجية يدعم فرضية التحول املخاطي لطبقة to the cases reported in the literature. The morphologic خلف الصفاق املتوسطة واملسبوقة بتكوين كيسي اشتمالي والتي and immunohistochemical analysis observed in this تؤدي إلى حدوث أورام خلف الصفاق املخاطية. -

Coordination of Endothelial Cell Positioning and Fate Specification By
ARTICLE https://doi.org/10.1038/s41467-021-24414-z OPEN Coordination of endothelial cell positioning and fate specification by the epicardium Pearl Quijada 1,8, Michael A. Trembley1, Adwiteeya Misra1,2, Jacquelyn A. Myers 3,4, Cameron D. Baker 3,4, Marta Pérez-Hernández 5, Jason R. Myers3,4, Ronald A. Dirkx Jr.1, ✉ Ethan David Cohen6, Mario Delmar5, John M. Ashton 3,4 & Eric M. Small 1,2,7 The organization of an integrated coronary vasculature requires the specification of immature 1234567890():,; endothelial cells (ECs) into arterial and venous fates based on their localization within the heart. It remains unclear how spatial information controls EC identity and behavior. Here we use single-cell RNA sequencing at key developmental timepoints to interrogate cellular contributions to coronary vessel patterning and maturation. We perform transcriptional profiling to define a heterogenous population of epicardium-derived cells (EPDCs) that express unique chemokine signatures. We identify a population of Slit2+ EPDCs that emerge following epithelial-to- mesenchymal transition (EMT), which we term vascular guidepost cells. We show that the expression of guidepost-derived chemokines such as Slit2 are induced in epicardial cells undergoing EMT, while mesothelium-derived chemokines are silenced. We demonstrate that epicardium-specific deletion of myocardin-related transcription factors in mouse embryos disrupts the expression of key guidance cues and alters EPDC-EC signaling, leading to the persistence of an immature angiogenic EC identity and inappropriate accumulation of ECs on the epicardial surface. Our study suggests that EC pathfinding and fate specification is controlled by a common mechanism and guided by paracrine signaling from EPDCs linking epicardial EMT to EC localization and fate specification in the developing heart.