Contrasting Patterns of Intercontinental Connectivity and Climatic Niche Evolution in “Terebinthaceae” (Anacardiaceae and Burseraceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Isolation and Characterization of Microsatellite Loci for the Large
Isolation and Characterization of Microsatellite Loci for the Large-Seeded Tree Protorhus deflexa (Anacardiaceae) Author(s): Hiroki Sato, Christopher Adenyo, Tsuyoshi Harata, Satoshi Nanami , Akira Itoh , Yukio Takahata , and Miho Inoue-Murayama Source: Applications in Plant Sciences, 1(12) 2013. Published By: Botanical Society of America DOI: http://dx.doi.org/10.3732/apps.1300046 URL: http://www.bioone.org/doi/full/10.3732/apps.1300046 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Applications Applications in Plant Sciences 2014 2 ( 1 ): 1300046 in Plant Sciences P RIMER NOTE I SOLATION AND CHARACTERIZATION OF MICROSATELLITE LOCI FOR THE LARGE-SEEDED -

The Condensed Tannins of Okoume (Aucoumea Klaineana Pierre)
www.nature.com/scientificreports OPEN The condensed tannins of Okoume (Aucoumea klaineana Pierre): A molecular structure and thermal stability study Starlin Péguy Engozogho Anris 1,2*, Arsène Bikoro Bi Athomo1,2, Rodrigue Safou Tchiama2,3, Francisco José Santiago-Medina4, Thomas Cabaret1, Antonio Pizzi4 & Bertrand Charrier1 In order to promote convenient strategies for the valorization of Aucoumea klaineana Pierre (Okoume) plywood and sawmill wastes industry in the felds of adhesives and composites, the total phenolic content of Okoume bark, sapwood and heartwood was measured. The molecular structure of tannins extracted from the bark was determined by Matrix Assisted Laser Desorption/Ionization Time-Of-Flight (Maldi-ToF) mass spectrometry and Fourier transform infrared spectroscopy (FTIR). The total phenolic content displayed signifcant diference (p = 0.001) between the bark, sapwood and heartwood which decreased as follows: 6 ± 0.4, 2 ± 0.8 and 0.7 ± 0.1% respectively. The pro-anthocyanidins content was also signifcantly diferent (p = 0.01) among the three wood wastes, and the bark was the richest in condensed tannins (4.2 ± 0.4%) compared to the sapwood (0.5 ± 0.1%) and heartwood (0.2 ± 0.2%). Liquid chromatography coupled mass spectroscopy (LC-MS) and Maldi-ToF analysis of the bark showed for the frst time that Okoume condensed tannins are fsetinidin, gallocatechin and trihydroxyfavan based monomers and complex polymers obtained with glycosylated units. No free catechin or robitinidin units were detected, whereas distinctive dihydroxy or trihydroxyfavan-3-benzoate dimers were observed in the investigated condensed tannin extracts. FTIR analysis showed the occurrence of glucan- and mannan-like sugars in the condensed tannins, and Maldi-ToF highlighted that these sugars should account for ten glycosylated units chemically bonded with two fsetinidins and one gallocatechin trimer. -

Seeds and Plants Imported
y ... - Issued July 26, 191$ U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. WILLIAM A. TAYLOR, Chief of Bureau. INVENTORY OF SEEDS AND PLANTS IMPORTED BY THE OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM JULY 1 TO SEPTEMBER 30, 1915. (No. 44; Nos. 4089G TO 41314.) "WASHINGTON: GOVERNMENT PRINTING OFFICE. 1918. Issued July 26,1918. U. S. DEPARTMENT OF AGRICULTURE, BUREAU OF PLANT INDUSTRY. WILLIAM A. TAYLOR, Chief of Bureau. INVENTORY OF SEEDS AND PLANTS IMPORTED OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM JULY 1 TO SEPTEMBER 30, 1915. (No. 44; Nos. 40896 TO 41314.) WASHINGTON: GOVERNMENT PRINTING OFFICE. 1918. BUREAU OF PLANT INDUSTRY. Chief of Bureau, WILLIAM A. TAYLOR. Associate Chief of Bureau, KARL P. KELLBRMAN. Officer in Charge of Publications, J. E. ROCKWELL, Chief Clerk, JAMES E. JONES. FOREIGN SEED AND PLANT INTRODUCTION. SCIENTIFIC STAPF. David Fairchild, Agricultural Explorer in Charge, P. H. Dorsett, Plant Introducer, in Charge of Plant Introduction Field Stations. B. T. Galloway, Plant Pathologist, in Charge of Plant Protection and Plant Propagation. Peter Bisset, Plant Introducer, in Charge of Foreign Plant Distribution. Frank N. Meyer, Wilson Popenoe, and F. C. Reimer, Agricultural Explorers. H. C. Skeels, S. C. Stuntz, and R. A. Young, Botanical Assistants. Henry E. Allanson, D. A. Bisset, R. N. Jones, P. G. Russell, and G. P. Van Eseltine, Scientific Assistants. Robert L. Beagles, Superintendent, Plant Introduction Field Station, Chico, Cal. E. O. Orpet, Assistant in Plant Introduction. Edward Simmonds, Superintendent, Plant Introduction Field Station, Miami, Fla. John M. Rankin, Superintendent, Yarrow Plant Introduction Field Station, Rockville, Md. -

Araceae), with P
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.05.326850; this version posted October 7, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Taxonomic revision of the threatened African genus Pseudohydrosme Engl. (Araceae), with P. ebo, a new, Critically Endangered species from Ebo, Cameroon. Martin Cheek¹, Barthelemy Tchiengue2, Xander van der Burgt¹ ¹Science, Royal Botanic Gardens, Kew, Richmond, Surrey, U.K. 2 IRAD-Herbier National Camerounais, Yaoundé, BP 1601, Cameroon Corresponding author: Martin Cheek¹ Email address: [email protected] ABSTRACT This is the first revision in nearly 130 years of the African genus Pseudohydrosme, formerly considered endemic to Gabon. Sister to Anchomanes, Pseudohydrosme is distinct from Anchomanes because of its 2–3-locular ovary (not unilocular), peduncle concealed by cataphylls at anthesis and far shorter than the spathe (not exposed, far exceeding the spathe), stipitate fruits and viviparous (vegetatively apomictic) roots (not sessile, roots non-viviparous). Three species, one new to science, are recognised, in two sections. Although doubt has previously been cast on the value of recognising Pseudohydrosme buettneri, of Gabon, it is here accepted and maintained as a distinct species in the monotypic section, Zyganthera. However, it is considered to be probably globally extinct. Pseudohydrosme gabunensis, type species of the genus, also Gabonese, is maintained in Sect. Pseudohydrosme together with Pseudohydrosme ebo sp.nov. of the Ebo Forest, Littoral, Cameroon, the first addition to the genus since the nineteenth century, and which extends the range of the genus 450 km north from Gabon, into the Cross-Sanaga biogeographic area. -

(Anacardium Excelsum) EN LA JURISDICCIÓN CAR
PLAN DE MANEJO Y CONSERVACIÓN DEL CARACOLÍ (Anacardium excelsum) EN LA JURISDICCIÓN CAR 2017 “PLAN DE MANEJO Y CONSERVACIÓN DEL CARACOLÍ (Anacardium excelsum) EN LA JURISDICCIÓN CAR.” Corporación Autónoma Regional de Cundinamarca CAR Dirección de Modelamiento, Monitoreo y Laboratorio Ambiental DMMLA Grupo de Biodiversidad Bogotá, D.C. 2017 “PLAN DE MANEJO Y CONSERVACIÓN DEL CARACOLÍ (Anacardium excelsum) EN LA JURISDICCIÓN CAR.” Autor: Giovanny Andres Morales Mora – Ingeniero Forestal Plan de manejo y conservación del Caracolí (Anacardium excelsum) en la jurisdicción CAR. Giovanny Andres Morales Mora. Bogotá, Colombia: Corporación Autónoma Regional de Cundinamarca - CAR, 2016. Corporación Autónoma Regional de Cundinamarca – CAR Revisión técnica: John Eduard Rojas Rojas Cartografía: Lina María Porte Martínez Fotografía: Giovanny A. Morales Mora y Juan Camilo Duque Yate. Disponible en: Corporación Autónoma Regional de Cundinamarca – CAR Carrera 7 36-45 Bogotá D.C., Colombia. Tel: 3209000 www.car.gov.co Cítese como: Morales G.A. 2016. Plan de manejo y conservación del Caracolí (Anacardium excelsum) en la jurisdicción CAR. Bogotá, Colombia, 47 pp. 2 Contenido INTRODUCCIÓN ........................................................................................................................ 6 1. CONTEXTO GENERAL ....................................................................................................... 7 1.1. Familia Anacardiaceae en Colombia............................................................................ 7 1.2. Géneros -

2. ACER Linnaeus, Sp. Pl. 2: 1054. 1753. 枫属 Feng Shu Trees Or Shrubs
Fl. China 11: 516–553. 2008. 2. ACER Linnaeus, Sp. Pl. 2: 1054. 1753. 枫属 feng shu Trees or shrubs. Leaves mostly simple and palmately lobed or at least palmately veined, in a few species pinnately veined and entire or toothed, or pinnately or palmately 3–5-foliolate. Inflorescence corymbiform or umbelliform, sometimes racemose or large paniculate. Sepals (4 or)5, rarely 6. Petals (4 or)5, rarely 6, seldom absent. Stamens (4 or 5 or)8(or 10 or 12); filaments distinct. Carpels 2; ovules (1 or)2 per locule. Fruit a winged schizocarp, commonly a double samara, usually 1-seeded; embryo oily or starchy, radicle elongate, cotyledons 2, green, flat or plicate; endosperm absent. 2n = 26. About 129 species: widespread in both temperate and tropical regions of N Africa, Asia, Europe, and Central and North America; 99 species (61 endemic, three introduced) in China. Acer lanceolatum Molliard (Bull. Soc. Bot. France 50: 134. 1903), described from Guangxi, is an uncertain species and is therefore not accepted here. The type specimen, in Berlin (B), has been destroyed. Up to now, no additional specimens have been found that could help clarify the application of this name. Worldwide, Japanese maples are famous for their autumn color, and there are over 400 cultivars. Also, many Chinese maple trees have beautiful autumn colors and have been cultivated widely in Chinese gardens, such as Acer buergerianum, A. davidii, A. duplicatoserratum, A. griseum, A. pictum, A. tataricum subsp. ginnala, A. triflorum, A. truncatum, and A. wilsonii. In winter, the snake-bark maples (A. davidii and its relatives) and paper-bark maple (A. -

Boissiera 71
Taxonomic treatment of Abrahamia Randrian. & Lowry, a new genus of Anacardiaceae BOISSIERA from Madagascar Armand RANDRIANASOLO, Porter P. LOWRY II & George E. SCHATZ 71 BOISSIERA vol.71 Director Pierre-André Loizeau Editor-in-chief Martin W. Callmander Guest editor of Patrick Perret this volume Graphic Design Matthieu Berthod Author instructions for www.ville-ge.ch/cjb/publications_boissiera.php manuscript submissions Boissiera 71 was published on 27 December 2017 © CONSERVATOIRE ET JARDIN BOTANIQUES DE LA VILLE DE GENÈVE BOISSIERA Systematic Botany Monographs vol.71 Boissiera is indexed in: BIOSIS ® ISSN 0373-2975 / ISBN 978-2-8277-0087-5 Taxonomic treatment of Abrahamia Randrian. & Lowry, a new genus of Anacardiaceae from Madagascar Armand Randrianasolo Porter P. Lowry II George E. Schatz Addresses of the authors AR William L. Brown Center, Missouri Botanical Garden, P.O. Box 299, St. Louis, MO, 63166-0299, U.S.A. [email protected] PPL Africa and Madagascar Program, Missouri Botanical Garden, P.O. Box 299, St. Louis, MO, 63166-0299, U.S.A. Institut de Systématique, Evolution, Biodiversité (ISYEB), UMR 7205, Centre national de la Recherche scientifique/Muséum national d’Histoire naturelle/École pratique des Hautes Etudes, Université Pierre et Marie Curie, Sorbonne Universités, C.P. 39, 57 rue Cuvier, 75231 Paris CEDEX 05, France. GES Africa and Madagascar Program, Missouri Botanical Garden, P.O. Box 299, St. Louis, MO, 63166-0299, U.S.A. Taxonomic treatment of Abrahamia (Anacardiaceae) 7 Abstract he Malagasy endemic genus Abrahamia Randrian. & Lowry (Anacardiaceae) is T described and a taxonomic revision is presented in which 34 species are recog- nized, including 19 that are described as new. -

Cintia Luz.Pdf
Cíntia Luíza da Silva Luz Filogenia e sistemática de Schinus L. (Anacardiaceae), com revisão de um clado endêmico das matas nebulares andinas Phylogeny and systematics of Schinus L. (Anacardiaceae), with revision of a clade endemic to the Andean cloud forests Tese apresentada ao Instituto de Biociências da Universidade de São Paulo, para obtenção de Título de Doutor em Ciências, na Área de Botânica. Orientador: Dr. José Rubens Pirani São Paulo 2017 Luz, Cíntia Luíza da Silva Filogenia e sistemática de Schinus L. (Anacardiaceae), com revisão de um clado endêmico das matas nebulares andinas Número de páginas: 176 Tese (Doutorado) - Instituto de Biociências da Universidade de São Paulo. Departamento de Botânica. 1. Anacardiaceae 2. Schinus 3. Filogenia 4. Taxonomia vegetal I. Universidade de São Paulo. Instituto de Biociências. Departamento de Botânica Comissão julgadora: ______________________________ ______________________________ Prof(a). Dr.(a) Prof(a). Dr.(a) ______________________________ ______________________________ Prof(a). Dr.(a) Prof(a). Dr.(a) _____________________________________ Prof. Dr. José Rubens Pirani Orientador Ao Luciano Luz, pelo entusiasmo botânico, companheirismo e dedicação aos Schinus Esta é a estória. Ia um menino, com os tios, passar dias no lugar onde se construía a grande cidade. Era uma viagem inventada no feliz; para ele, produzia-se em caso de sonho. Saíam ainda com o escuro, o ar fino de cheiros desconhecidos. A mãe e o pai vinham trazê-lo ao aeroporto. A tia e o tio tomavam conta dele, justínhamente. Sorria-se, saudava-se, todos se ouviam e falavam. O avião era da companhia, especial, de quatro lugares. Respondiam-lhe a todas as perguntas, até o piloto conversou com ele. -

Paperbark Maple Acer Griseum Paul W
During NACPEC expeditions plant species are targeted for collection for a range of reasons including environmental adaptabilities, conservation value, and ornamental features. Presented here are thirteen profiles of notable plants collected on these expeditions. Paperbark Maple Acer griseum Paul W. Meyer aperbark maple is an iconic Chinese spe- The paperbark maples growing on Wudang cies with beautiful exfoliating cinna- Mountain were relatively small, growing on a Pmon-colored bark that never fails to grab west-facing slope in thin, rocky soil. Being in attention. It is frequently highlighted in public the understory, most were leggy and the foliage gardens and connoisseurs’ gardens throughout was high off the ground. With careful observa- the temperate world. It was first introduced to tion though, we spotted the winged samaras the United States by E. H. Wilson through the in the upper canopy. Using pole pruners, we Arnold Arboretum in 1907. were able to collect herbarium specimens and In addition to its stunning bark, this species a small seed sample. is widely admired for its clean, fine-textured The following year, in April 1995, NACPEC foliage, orange-red fall color, and relatively small team members Rick lewandowski, Teicheng stature, usually under 35 feet (10.7 meters) tall. Cui, and Ned Garvey spotted an incredible It is believed that until recently, all or most specimen of paperbark maple in the Baxiam paperbark maples in the United States derived Forest Station in Shaanxi, less than 200 kilo- from the genetically narrow 1907 Wilson intro- meters (124 miles) west of Wudang Mountain. duction—it consisted of only two plants. -

ISTA List of Stabilised Plant Names 7Th Edition
ISTA List of Stabilised Plant Names 7th Edition ISTA Nomenclature Committee Chair Dr. M. Schori Published by All rights reserved. No part of this publication may be The International Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted in Richtiarkade 18, CH- 8304 Wallisellen, Switzerland any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2021 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 Valid from: 16.06.2021 ISTA List of Stabilised Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 7th Edition 2 ISTA List of Stabilised Plant Names Table of Contents A .............................................................................................................................................................. 7 B ............................................................................................................................................................ 21 C ........................................................................................................................................................... -
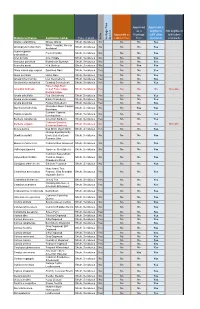
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila