DICOM Strategy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

X3DOM – Declarative (X)3D in HTML5
X3DOM – Declarative (X)3D in HTML5 Introduction and Tutorial Yvonne Jung Fraunhofer IGD Darmstadt, Germany [email protected] www.igd.fraunhofer.de/vcst © Fraunhofer IGD 3D Information inside the Web n Websites (have) become Web applications n Increasing interest in 3D for n Product presentation n Visualization of abstract information (e.g. time lines) n Enriching experience of Cultural Heritage data n Enhancing user experience with more Example Coform3D: line-up of sophisticated visualizations scanned historic 3D objects n Today: Adobe Flash-based site with videos n Tomorrow: Immersive 3D inside browsers © Fraunhofer IGD OpenGL and GLSL in the Web: WebGL n JavaScript Binding for OpenGL ES 2.0 in Web Browser n à Firefox, Chrome, Safari, Opera n Only GLSL shader based, no fixed function pipeline mehr n No variables from GL state n No Matrix stack, etc. n HTML5 <canvas> element provides 3D rendering context n gl = canvas.getContext(’webgl’); n API calls via GL object n X3D via X3DOM framework n http://www.x3dom.org © Fraunhofer IGD X3DOM – Declarative (X)3D in HTML5 n Allows utilizing well-known JavaScript and DOM infrastructure for 3D n Brings together both n declarative content design as known from web design n “old-school” imperative approaches known from game engine development <html> <body> <h1>Hello X3DOM World</h1> <x3d> <scene> <shape> <box></box> </shape> </scene> </x3d> </body> </html> © Fraunhofer IGD X3DOM – Declarative (X)3D in HTML5 • X3DOM := X3D + DOM • DOM-based integration framework for declarative 3D graphics -

TR 102 199 V1.1.1 (2003-10) Technical Report
ETSI TR 102 199 V1.1.1 (2003-10) Technical Report Services and Protocols for Advanced Networks (SPAN); Preliminary analysis of Broadband multimedia services 2 ETSI TR 102 199 V1.1.1 (2003-10) Reference DTR/SPAN-130320 Keywords broadband, multimedia, service ETSI 650 Route des Lucioles F-06921 Sophia Antipolis Cedex - FRANCE Tel.: +33 4 92 94 42 00 Fax: +33 4 93 65 47 16 Siret N° 348 623 562 00017 - NAF 742 C Association à but non lucratif enregistrée à la Sous-Préfecture de Grasse (06) N° 7803/88 Important notice Individual copies of the present document can be downloaded from: http://www.etsi.org The present document may be made available in more than one electronic version or in print. In any case of existing or perceived difference in contents between such versions, the reference version is the Portable Document Format (PDF). In case of dispute, the reference shall be the printing on ETSI printers of the PDF version kept on a specific network drive within ETSI Secretariat. Users of the present document should be aware that the document may be subject to revision or change of status. Information on the current status of this and other ETSI documents is available at http://portal.etsi.org/tb/status/status.asp If you find errors in the present document, send your comment to: [email protected] Copyright Notification No part may be reproduced except as authorized by written permission. The copyright and the foregoing restriction extend to reproduction in all media. © European Telecommunications Standards Institute 2003. All rights reserved. -

BIM & IFC-To-X3D
BIM & IFC-to-X3D Hyokwang Lee PartDB Co., Ltd. and Web3D Korea Chapter [email protected] Engineering IT & VR solutions based on International Standards BIM . BIM (Building Information Modeling) . A digital representation of physical and functional characteristics of a facility. A shared knowledge resource for information about a facility forming a reliable basis for decisions during its life-cycle; defined as existing from earliest conception to demolition. http://www.directindustry.com/ http://www.aecbytes.com/buildingthefuture/2007/BIM_Awards_Part1.html Coordination between the different disciplinary models. (© M.A. Mortenson Company) 4D scheduling using the BIM model (top image) and the details of site utilization and civil work (lower image) used for coordination and communication with local review agencies and utility companies. (© M.A. Mortenson Company) http://www.quartzsys.com/ Design http://www.quartzsys.com/?c=1/6/27 OpenBIM . A universal approach to the collaborative design, realization and operation of buildings based on open standards and workflows. An initiative of buildingSMART and several leading software vendors using the open buildingSMART Data Model. http://buildingsmart.com/openbim OpenBIM & IFC . IFC (ISO/PAS 16739) . The Industry Foundation Classes (IFC) : an open, neutral and standard ized specification for Building Information Models, BIM. The main buildingSMART data model standard buildingSMART Web3D Consortium International IFC Web3D (ISO/PAS 16739) Web3D Korea Chapter buildingSMART KOREA - Inhan Kim, What is BIM?, Aug. 2008, http://www.cadgraphics.co.kr/cngtv/data/20080813_1.pdf BIM CAD Systems . Autodesk Revit Architecture . Bently Achitecture . Nemetschek Allplan . GRAPHISOFT ArchiCad . Gehry Technologies (GT) Digital Project . CATIA V5 as a core modeling engine . Vectorworks Architect Simple IFC-to-X3D Conversion . -

ISO/IEC JTC 1 N13604 ISO/IEC JTC 1 Information Technology
ISO/IEC JTC 1 N13604 2017-09-17 Replaces: ISO/IEC JTC 1 Information Technology Document Type: other (defined) Document Title: Study Group Report on 3D Printing and Scanning Document Source: SG Convenor Project Number: Document Status: This document is circulated for review and consideration at the October 2017 JTC 1 meeting in Russia. Action ID: ACT Due Date: 2017-10-02 Pages: Secretariat, ISO/IEC JTC 1, American National Standards Institute, 25 West 43rd Street, New York, NY 10036; Telephone: 1 212 642 4932; Facsimile: 1 212 840 2298; Email: [email protected] Study Group Report on 3D Printing and Scanning September 11, 2017 ISO/IEC JTC 1 Plenary (October 2017, Vladivostok, Russia) Prepared by the ISO/IEC JTC 1 Study Group on 3D Printing and Scanning Executive Summary The purpose of this report is to assess the possible contributions of JTC 1 to the global market enabled by 3D Printing and Scanning. 3D printing, also known as additive manufacturing, is considered by many sources as a truly disruptive technology. 3D printers range presently from small table units to room size and can handle simple plastics, metals, biomaterials, concrete or a mix of materials. They can be used in making simple toys, airplane engine components, custom pills, large buildings components or human organs. Depending on process, materials and precision, 3D printer costs range from hundreds to millions of dollars. 3D printing makes possible the manufacturing of devices and components that cannot be constructed cost-effectively with other manufacturing techniques (injection molding, computerized milling, etc.). It also makes possible the fabrications of customized devices, or individual (instead of identical mass-manufactured) units. -

University of Florida Acceptable ETD Formats
University of Florida acceptable ETD formats The Acceptable ETD formats below are defined based on those media for which a preservation strategy exists. The aim is to make all ETD’s available into the future as the technology changes and software becomes obsolete. Media formats that are not listed in this table are ones that cannot presently be preserved at an acceptable level. Many of these can easily be converted to one of the acceptable formats. If you create a document using Word or LaTex it should be exported as a PDF. Please consult with the CIRCA ETD unit ([email protected]) for assistance or if you have any questions. The Acceptable ETD Formats are reviewed and updated regularly by the Graduate School, the Library, the ETD training staff in Academic Technology, and the Florida Center for Library Automation. All files should be scanned with up-to-date virus software before submission. Files with viruses will not be accepted. Media Acceptable formats (bold indicates preferred formats) Text - PDF/A-1 (ISO 19005-1) (*.pdf) - Plain text (encoding: USASCII, UTF-8, UTF-16 with BOM) - XML (includes XSD/XSL/XHTML, etc.; with included or accessible schema and character encoding explicitly specified) - Cascading Style Sheets (*.css) - DTD (*.dtd) - Plain text (ISO 8859-1 encoding) - PDF (*.pdf) (embedded fonts) - Rich Text Format 1.x (*.rtf) - HTML (include a DOCTYPE declaration) - SGML (*.sgml) - Open Office (*.sxw/*.odt) - OOXML (ISO/IEC DIS 29500) (*.docx) - Computer program source code (*.c, *.c++, *.java, *.js, *.jsp, *.php, *.pl, etc.) -

Forcepoint DLP Supported File Formats and Size Limits
Forcepoint DLP Supported File Formats and Size Limits Supported File Formats and Size Limits | Forcepoint DLP | v8.8.1 This article provides a list of the file formats that can be analyzed by Forcepoint DLP, file formats from which content and meta data can be extracted, and the file size limits for network, endpoint, and discovery functions. See: ● Supported File Formats ● File Size Limits © 2021 Forcepoint LLC Supported File Formats Supported File Formats and Size Limits | Forcepoint DLP | v8.8.1 The following tables lists the file formats supported by Forcepoint DLP. File formats are in alphabetical order by format group. ● Archive For mats, page 3 ● Backup Formats, page 7 ● Business Intelligence (BI) and Analysis Formats, page 8 ● Computer-Aided Design Formats, page 9 ● Cryptography Formats, page 12 ● Database Formats, page 14 ● Desktop publishing formats, page 16 ● eBook/Audio book formats, page 17 ● Executable formats, page 18 ● Font formats, page 20 ● Graphics formats - general, page 21 ● Graphics formats - vector graphics, page 26 ● Library formats, page 29 ● Log formats, page 30 ● Mail formats, page 31 ● Multimedia formats, page 32 ● Object formats, page 37 ● Presentation formats, page 38 ● Project management formats, page 40 ● Spreadsheet formats, page 41 ● Text and markup formats, page 43 ● Word processing formats, page 45 ● Miscellaneous formats, page 53 Supported file formats are added and updated frequently. Key to support tables Symbol Description Y The format is supported N The format is not supported P Partial metadata -
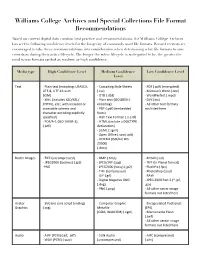
Williams College Archives and Special Collections File Format Recommendations
Williams College Archives and Special Collections File Format Recommendations Based on current digital data curation best practice and recommendations, the Williams College Archives has set the following confidence levels for the longevity of commonly used file formats. Record creators are encouraged to take these recommendations into consideration when determining what file formats to save records as during their active lifecycle. The longer the active lifecycle is anticipated to be, the greater the need to use formats ranked as medium or high confidence. Media type High Confidence Level Medium Confidence Low Confidence Level Level Text - Plain text (encoding: USASCII, - Cascading Style Sheets - PDF (.pdf) (encrypted) UTF-8, UTF-16 with (.css) - Microsoft Word (.doc) BOM) - DTD (.dtd) - WordPerfect (.wpd) - XML (includes XSD/XSL/ - Plain text (ISO 8859-1 - DVI (.dvi) XHTML, etc.; with included or encoding) - All other text forMats accessible schema and - PDF (.pdf) (eMbedded not listed here character encoding explicitly fonts) specified) - Rich Text ForMat 1.x (.rtf) - PDF/A-1 (ISO 19005-1) - HTML (include a DOCTYPE (.pdf) declaration) - SGML (.sgMl) - Open Office (.sxw/.odt) - OOXML (ISO/IEC DIS 29500) (.docx) Raster Images - TIFF (uncoMpressed) - BMP (.bMp) - MrSID (.sid) - JPEG2000 (lossless) (.jp2) - JPEG/JFIF (.jpg) - TIFF (in Planar forMat) -PNG - JPEG2000 (lossy) (.jp2) - FlashPix (.fpx) - TIFF (coMpressed) - PhotoShop (.psd) - GIF (.gif) - RAW - Digital Negative DNG - JPEG 2000 Part 2 (*.jpf, (.dng) .jpx) - PNG (.png) - All other -

Technical Overview
Brutzman-Ch01.qxd 2/16/07 12:27 PM Page 1 1 CHAPTER Technical Overview When we mean to build, we first survey the plot, then draw the model. —William Shakespeare, Henry IV Part II Act 1 Scene 2 1. Introduction Building and interacting with 3D graphics is a “hands on” experience. There are many examples in this book to teach you how X3D works and to assist you in building your own projects. However, before creating your own Extensible 3D (X3D) graphics scenes, you should understand the background concepts explained here. The book has an accompanying website at X3dGraphics.com. All examples plus links to other reference material and software are available on the website. This chapter presents the ideas needed to understand how an X3D scene graph works. This information is used throughout the following chapters and is especially helpful when creating your own X3D scenes. This book assumes that you are interested in learning more about 3D graphics—prior knowledge is helpful, but not required. This chapter is best for people who already have some knowledge of 3D graphics and are ready to learn more of the technical background about how X3D works. X3D uses a scene graph to model the many graphics nodes that make up a virtual environment. The scene graph is a tree structure that is directed and acyclic, meaning 1 Brutzman-Ch01.qxd 2/16/07 12:27 PM Page 2 2 CHAPTER 1 Technical Overview that there is a definite beginning for the graph, there are parent-child relationships for each node, and there are no cycles (or loops) in the graph. -

{Download PDF} X3D : Extensible 3D Graphics for Web Authors Ebook
X3D : EXTENSIBLE 3D GRAPHICS FOR WEB AUTHORS PDF, EPUB, EBOOK Don Brutzman | 472 pages | 04 May 2007 | ELSEVIER SCIENCE & TECHNOLOGY | 9780120885008 | English | San Francisco, United States X3D : Extensible 3D Graphics for Web Authors PDF Book This Collection. Cerca Negozio. Such capabilities can also be implemented directly by a software renderer when such hardware-driven graphics acceleration is not available. Sposta il mouse per ingrandire - Clicca per ingrandire. Expressing 3D within the domain of Extensible Markup Language XML for the Web is novel and has the potential to open up computer graphics to many new practitioners. Launch Research Feed. It includes simple animation interpolation but not user interaction. These documents are split into parts, each of which addresses a specific topic. We believe that it is a travesty to simply throw away a used book when there is nothing wrong with it - we believe in giving each book the chance of finding a new home. The test suite is initially self-administered by implementing company members, and test results are formally verified by the Web3D Consortium. However, due to transit disruptions in some geographies, deliveries may be delayed. Info sull'oggetto Condizione:. By Johannes Behr. Typically, there are updates for three or four of these parts undergoing the ISO review process at any given time. World of Books Ltd sells quality used books at competitive prices to over 2 million customers worldwide each year. X3D is designed so that all of these forms are functionally equivalent, you can choose to use any of them. Resources include a detailed textbook, an authoring tool, hundreds of example scenes, and detailed slidesets covering each chapter. -

IDOL Keyview Viewing SDK 12.7 Programming Guide
KeyView Software Version 12.7 Viewing SDK Programming Guide Document Release Date: October 2020 Software Release Date: October 2020 Viewing SDK Programming Guide Legal notices Copyright notice © Copyright 2016-2020 Micro Focus or one of its affiliates. The only warranties for products and services of Micro Focus and its affiliates and licensors (“Micro Focus”) are set forth in the express warranty statements accompanying such products and services. Nothing herein should be construed as constituting an additional warranty. Micro Focus shall not be liable for technical or editorial errors or omissions contained herein. The information contained herein is subject to change without notice. Documentation updates The title page of this document contains the following identifying information: l Software Version number, which indicates the software version. l Document Release Date, which changes each time the document is updated. l Software Release Date, which indicates the release date of this version of the software. To check for updated documentation, visit https://www.microfocus.com/support-and-services/documentation/. Support Visit the MySupport portal to access contact information and details about the products, services, and support that Micro Focus offers. This portal also provides customer self-solve capabilities. It gives you a fast and efficient way to access interactive technical support tools needed to manage your business. As a valued support customer, you can benefit by using the MySupport portal to: l Search for knowledge documents of interest l Access product documentation l View software vulnerability alerts l Enter into discussions with other software customers l Download software patches l Manage software licenses, downloads, and support contracts l Submit and track service requests l Contact customer support l View information about all services that Support offers Many areas of the portal require you to sign in. -

X3D Graphics and Distributed Interactive Simulation
Calhoun: The NPS Institutional Archive Faculty and Researcher Publications Faculty and Researcher Publications 2014-08 DIY X3D, Do It Yourself X3D Graphics! Brutzman, Don http://hdl.handle.net/10945/46002 DIY X3D, Do It Yourself X3D Graphics! Web3D 2014 Conference Vancouver Canada 8-10 August 2014 Don Brutzman Naval Postgraduate School [email protected] What is Extensible 3D (X3D)? 3D publishing language for Web What is Extensible 3D (X3D)? X3D is a royalty-free open-standard file format • Communicate animated 3D scenes using XML • Run-time architecture for consistent user interaction • ISO-ratified standard for storage, retrieval and playback of real-time graphics content • Enables real-time communication of 3D data across applications: archival publishing format for Web • Rich set of componentized features for engineering and scientific visualization, CAD and architecture, medical visualization, training and simulation, multimedia, entertainment, education, and more Bottom Line Up Front (BLUF) X3D-Edit is open-source tool we use and build to create, modify, test, publish, modify scenes :' XJO£diIJ]]OO711 ' J']OO ~ o x r.... ..........C ..;!,._.... ;;. ,;o>o,. -I I ~fonna t lon ' I ~ __ ""'TC·-:'"~·__ -=~~C"~C-\P=-c<b~~~o-S5~7~;.-"41~:c __ =-"~C-_~~ ________________________________________________ __ 6 Ceometry - Prirrltlves ! <Ix.). vtt lu.o ll"'''' l , 0 " e lKo<UD9"' ''l11T- S"', > iii .0 ""'" • eox 4 cone • ~ < ! II"CIYPE Xl1I P\J8LIC "110/llIeo l OI i PTO XlP 'J , : I/u" "http. II_ , _0]d,otIJ/ . peo;o l.f l.Cat1.01IlIII / lI1d· ] ,:;: . dtd"> ,. Text , Fcnt Styte , <x)P profJ.l~' ~ r=- l.vt ' verll.u>D"' ' '): .: ' 1CllIl= ; lliJdK ' http. -

Web-Enabled Intelligent System for Real-Time, Continuous Thermal
Web-enabled Intelligent System for Continuous Sensor Data Processing and Visualization Felix G. Hamza-Lup Ionut E. Iacob Sushmita Khan Computer Science Mathematical Sciences Mathematical Sciences Georgia Southern University Georgia Southern University Georgia Southern University Savannah GA US Statesboro, GA, USA Statesboro GA US [email protected] [email protected] [email protected] ABSTRACT A large number of sensors deployed in recent years in various 1 Introduction setups and their data is readily available in dedicated databases or The widespread of sensors in the IoT context and the emergence in the cloud. Of particular interest is real-time data processing and of Web3D standards, enable big data collection and visualization, 3D visualization in web-based user interfaces that facilitate spatial empowering Internet collaborations among participants with information understanding and sharing, hence helping the decision different backgrounds, and facilitating the decision process making process for all the parties involved. through spatial data and complex information understanding. In this research, we provide a prototype system for near real- We employ the X3D ISO standard [1] and the Finite time, continuous X3D-based visualization of processed sensor Difference Method (FDM) computational model with a Machine data for two significant applications: thermal monitoring for Learning (ML) based enhancement, to process data from a set of residential/commercial buildings and nitrogen cycle monitoring in sensors attached at fixed locations in the monitored environment. water beds for aquaponics systems. As sensors are sparsely The proposed sensor data processing allows generation of placed, in each application, where they collect data for large meaningful supplementary data in the entire monitored volume, to periods (of up to one year), we employ a Finite Differences enable generation of X3D-based colormaps with variable Method and a Neural Networks model to approximate data transparency values (α- values).