First Isolation of Virulent Tenacibaculum Maritimum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Field Guide to the Nonindigenous Marine Fishes of Florida
Field Guide to the Nonindigenous Marine Fishes of Florida Schofield, P. J., J. A. Morris, Jr. and L. Akins Mention of trade names or commercial products does not constitute endorsement or recommendation for their use by the United States goverment. Pamela J. Schofield, Ph.D. U.S. Geological Survey Florida Integrated Science Center 7920 NW 71st Street Gainesville, FL 32653 [email protected] James A. Morris, Jr., Ph.D. National Oceanic and Atmospheric Administration National Ocean Service National Centers for Coastal Ocean Science Center for Coastal Fisheries and Habitat Research 101 Pivers Island Road Beaufort, NC 28516 [email protected] Lad Akins Reef Environmental Education Foundation (REEF) 98300 Overseas Highway Key Largo, FL 33037 [email protected] Suggested Citation: Schofield, P. J., J. A. Morris, Jr. and L. Akins. 2009. Field Guide to Nonindigenous Marine Fishes of Florida. NOAA Technical Memorandum NOS NCCOS 92. Field Guide to Nonindigenous Marine Fishes of Florida Pamela J. Schofield, Ph.D. James A. Morris, Jr., Ph.D. Lad Akins NOAA, National Ocean Service National Centers for Coastal Ocean Science NOAA Technical Memorandum NOS NCCOS 92. September 2009 United States Department of National Oceanic and National Ocean Service Commerce Atmospheric Administration Gary F. Locke Jane Lubchenco John H. Dunnigan Secretary Administrator Assistant Administrator Table of Contents Introduction ................................................................................................ i Methods .....................................................................................................ii -

Disease of Aquatic Organisms 105:1
Vol. 105: 1–8, 2013 DISEASES OF AQUATIC ORGANISMS Published July 9 doi: 10.3354/dao02594 Dis Aquat Org Megalocytivirus infection in orbiculate batfish Platax orbicularis Preeyanan Sriwanayos1, Ruth Francis-Floyd1,2, Mark F. Stidworthy3, Barbara D. Petty1,2, Karen Kelley4, Thomas B. Waltzek5,* 1Program in Fisheries and Aquatic Sciences, School of Forestry Resources and Conservation, University of Florida, Gainesville, Florida 32653, USA 2Department of Large Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, Florida 32610, USA 3International Zoo Veterinary Group, Station House, Parkwood Street, Keighley, West Yorkshire BD21 4NQ, UK 4Interdisciplinary Center for Biotechnology Research (ICBR), Cellomics Division, Electron Microscopy and Bio-imaging Core Laboratory, University of Florida, Gainesville, Florida 32611, USA 5Department of Infectious Diseases and Pathology, College of Veterinary Medicine, University of Florida, Gainesville, Florida 32608, USA ABSTRACT: Megalocytiviruses cause systemic disease in both marine and freshwater fishes, neg- atively impacting ornamental and food fish aquaculture. In this report, we characterize a megalo- cytivirus infection in a captive marine ornamental fish, the orbiculate batfish Platax orbicularis. Histologic examination revealed cytomegalic cells characterized by strongly basophilic granular intracytoplasmic inclusions within various organs. Transmission electron microscopy revealed icosahedral virus particles within the cytoplasm of cytomegalic cells consistent -

The Malay Archipelago
BOOKS & ARTS COMMENT The Malay Archipelago: the land of the orang-utan, and the bird of paradise; a IN RETROSPECT narrative of travel, with studies of man and nature ALFRED RUSSEL WALLACE The Malay Macmillan/Harper Brothers: first published 1869. lfred Russel Wallace was arguably the greatest field biologist of the nine- Archipelago teenth century. He played a leading Apart in the founding of both evolutionary theory and biogeography (see page 162). David Quammen re-enters the ‘Milky Way of He was also, at times, a fine writer. The best land masses’ evoked by Alfred Russel Wallace’s of his literary side is on show in his 1869 classic, The Malay Archipelago, a wondrous masterpiece of biogeography. book of travel and adventure that wears its deeper significance lightly. The Malay Archipelago is the vast chain of islands stretching eastward from Sumatra for more than 6,000 kilometres. Most of it now falls within the sovereignties of Malaysia and Indonesia. In Wallace’s time, it was a world apart, a great Milky Way of land masses and seas and straits, little explored by Europeans, sparsely populated by peoples of diverse cul- tures, and harbouring countless species of unknown plant and animal in dense tropical forests. Some parts, such as the Aru group “Wallace paid of islands, just off the his expenses coast of New Guinea, by selling ERNST MAYR LIB., MUS. COMPARATIVE ZOOLOGY, HARVARD UNIV. HARVARD ZOOLOGY, LIB., MUS. COMPARATIVE MAYR ERNST were almost legend- specimens. So ary for their remote- he collected ness and biological series, not just riches. Wallace’s jour- samples.” neys throughout this region, sometimes by mail packet ship, some- times in a trading vessel or a small outrigger canoe, were driven by a purpose: to collect animal specimens that might help to answer a scientific question. -

Interplay Between Hormonal and Morphological Changes Throughout a Critical Period of Larval Rearing in the Orbicular Batfish
Aquaculture Reports 18 (2020) 100521 Contents lists available at ScienceDirect Aquaculture Reports journal homepage: www.elsevier.com/locate/aqrep Interplay between hormonal and morphological changes throughout a critical period of larval rearing in the orbicular batfish Viliame Waqalevu a,b,c,1, Marc Besson c,d,1, Camille Gache c,1, Natacha Roux c,e, Lily Fogg f, Fr´ederic´ Bertucci g,h, Marc Metian d, Marc-Andr´e Lafille i, Moana Maamaatuaiahutapu i, Eric Gasset j, Denis Saulnier k,l, Vincent Laudet e, David Lecchini c,l,* a Laboratory of Larval Rearing Management, United Graduate School of Agricultural Sciences, 8908580, Kagoshima University, Japan b School of Marine Studies, University of the South Pacific, 15387, Suva, Fiji c PSL Research University, EPHE-UPVD-CNRS, USR 3278 CRIOBE BP 1013, 98729, Papetoai, Moorea, French Polynesia d International Atomic Energy Agency - Environment Laboratories, 4a Quai Antoine 1er, Monaco e Observatoire Oc´eanologique de Banyuls-sur-Mer, UMR CNRS 7232 BIOM, Sorbonne Universit´e, 1, avenue Pierre Fabre, 66650, Banyuls-sur-Mer, France f Queensland Brain Institute, University of Queensland, Brisbane, Australia g Unit´e FRE BOREA, MNHN, CNRS 7208, Sorbonne University, IRD 207, University Caen Normandy, University of French West Indies, Guadeloupe h Laboratoire de Morphologie Fonctionnelle et Evolutive, AFFISH-RC, University of Li`ege, Belgium i Direction des Ressources Marines, BP 20, Papeete, Tahiti, 98713, French Polynesia j MARBEC, Univ. Montpellier, CNRS, Ifremer, IRD, Palavas, France k Ifremer, UMR 241 EIO, UPF-ILM-IRD, B.P. 49, 98719, Taravao, Tahiti, French Polynesia l Laboratoire d’Excellence "CORAIL", 98729 Papetoai, Moorea, French Polynesia ARTICLE INFO ABSTRACT Keywords: Advancement and diversification of the aquaculture industry is reliant on the development of captive breeding Larval rearing and rearing protocols for novel fish species. -

ABDOU A., KEITH P., GALZIN R., 2015- Freshwater Neritids (Mollusca : Gastropoda) of Tropical Island, Amphidromy As a Life Cycle, a Review
ABDOU A., KEITH P., GALZIN R., 2015- Freshwater neritids (mollusca : Gastropoda) of tropical island, amphidromy as a life cycle, a review. Revue d’Ecologie (Terre et Vie), 70 (4) : 387-397. ADJEROUD M., AUGUSTIN D., GALZIN R., SALVAT B., 2002- Natural disturbances and interannual variability of coral reef communities on the outer slope of Tiahura (Moorea, French Polynesia): 1991 to 1997. Mar. Ecol. Progr. Ser. 237 : 121-131 ADJEROUD M., CHANCERELLE Y., SCHRIMM M., PEREZ T., LECCHINI D., GALZIN R., SALVAT B., 2005 – Detecting the effects of natural disturbances on coral assemblages in French Polynesia : a decade survey at multiple scales. Aquatic Living resources, 18 : 111-123. ADJEROUD M., MICHONNEAU F., EDMUNDS P., CHANCERELLE Y., LISON DE LOMA T., PENIN L., THIBAUT L., VIDAL-DUPIOL J., SALVAT B., GALZIN R., 2009- Recurrent disturbances, recovery trajectories and resilience of coral assemblages on a south central pacific. Reef. Coral Reefs, 28 : 775-780. AMANIEU M., DO CHI T., GALZIN R., LASSERRE G., 1978 - Structure et importance des populations de crabe vert Carcinus mediterraneus Czerniavsky, 1884, dans l'étang du Prévost (Languedoc - France). Bull. Ecol., 9 (4): 329-341. ARIAS-GONZALEZ E., HERTEL O., GALZIN R., 1998 - Fonctionnement trophique d'un écosystème récifal en Polynésie française. Cybium, 22(2) : 1-24 ARIAS-GONZALEZ E., NUNEZ-LARA E., GONZALEZ-SALAS C., GALZIN R., 2004- Trophic models for investigation of fishing effect on coral reef ecosystems. Ecological Modeling. 172: 197-212 ARIAS-GONZALEZ E.,.GALZIN R., HARMELIN-VIVIEN M., 2004- Spatial, ontogenetic and temporal variation in the feeding habits of the squirrelfish Sargocentron microstoma on reefs in Moorea, French Polynesia. -

Platax Teira (Forsskål, 1775)
Platax teira (Forsskål, 1775) Rema Madhu and K. Madhu IDENTIFICATION Order : Perciformes Family : Ephippidae Common/FAO Name (English) : Longfin batfish Local names:names Not available MORPHOLOGICAL DESCRIPTION Body is deep and compressed; body depth is 0.9-1.2 times standard length of fish. Juveniles are also deep bodied with very long pelvic fins and long anal and dorsal fins (which shorten on becoming adults). Fins are elevated in both adults and juveniles. The fish is covered with small, ctenoid scales. It has a terminal mouth with bands of tricuspid teeth. Adults are silver-grey in colour, with a dark band through the eye extending to origin of pelvic fin and from base of dorsal fin origin to belly. A black blotch may be present at the terminus of the second band. A small black vertical streak is often present at origin of anal fin. Median fins are with black margins posteriorly. Five pores are on each side of lower jaw. Preopercle is smooth and opercle is without spines. Dorsal spines (total): 5-6; dorsal soft rays (total): 28-37; anal spines: 3 and anal soft rays: 22-28. Source of image : CMFRI, Kochi 349 PROFILE GEOGRAPHICAL DISTRIBUTION Platax teira is distributed in tropical and subtropical waters of the Indo-West Pacific region from the Red Sea to South Africa, Japan (Hokkaido), Taiwan Province of China, Philippines, Indonesia, New Guinea, northern Australia and Melanesia. It is also reported from Bay of Islands, New Zealand and Persian Gulf. HABITAT AND BIOLOGY Adults are found in sheltered bays, offshore areas, lagoons and seaward reefs. -
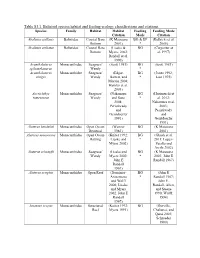
Table S3.1. Balistoid Species Habitat and Feeding Ecology Classifications and Citations
Table S3.1. Balistoid species habitat and feeding ecology classifications and citations. Species Family Habitat Habitat Feeding Feeding Mode Citation Mode Citation Abalistes stellaris Balistidae Coastal Bare (K Matsuura BG & EP (Kulbicki et al. Bottom 2001) * 2005) Abalistes stellatus Balistidae Coastal Bare (Lieske & BG (Carpenter et Bottom Myers, 2002; al. 1997) Randall et al. 1990) Acanthaluteres Monacanthidae Seagrass/ (Scott 1981) BG (Scott 1981) spilomelanurus Weedy * Acanthaluteres Monacanthidae Seagrass/ (Edgar, BG (Jones 1992; vittiger Weedy Barrett, and * Last 1975) Morton 2004; Hyndes et al. 2003) Acreichthys Monacanthidae Seagrass/ (Nakamura BG (Horinouchi et tomentosus Weedy and Sano * al. 2012; 2004; Nakamura et al. Peristiwady 2003; and Peristiwady Geistdoerfer and 1991) Geistdoerfer 1991) Aluterus heudeloti Monacanthidae Open Ocean (Wenner BG (K Matsuura Demersal 1983) 2001) Aluterus monoceros Monacanthidae Open Ocean (Kuiter 1992; BG (Ghosh et al. Rafting Lieske and * 2011; Lopez- Myers 2002) Peralta and Arcila 2002) Aluterus schoepfii Monacanthidae Seagrass/ (Lieske and BG (K Matsuura Weedy Myers 2002; * 2001; John E John E Randall 1967) Randall 1967) Aluterus scriptus Monacanthidae Open Reef (Dominici- BG (John E Arosemena * Randall 1967; and Wolff John E. 2006; Lieske Randall, Allen, and Myers and Steene 2002; John E 1990; Wulff, Randall 1994) 1967) Amanses scopas Monacanthidae Structured (Kuiter 1992; BG (Durville, Reef Myers 1991) Chabanet, and Quod 2003; Schroeder 1980) Balistapus Balistidae Structured (Hiatt and BG & EP (Hiatt and undulatus Reef Strasburg * Strasburg 1960; 1960; Lieske John E. Randall and Myers 1955; John E. 2002; Randall, Allen, McClanahan and Steene and Shafir 1990) 1990; John E. Randall, Allen, and Steene 1990) Balistes capriscus Balistidae Open Ocean (Longley BG & EP (Goldman, Pelagic 1941) * Glasgow, and Falk 2016; Lieske and Myers 2002; Vose and Nelson 1994) Balistes polylepis Balistidae Open Reef (Burgess et BG & EP (Abitia al. -

Authorship, Availability and Validity of Fish Names Described By
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Stuttgarter Beiträge Naturkunde Serie A [Biologie] Jahr/Year: 2008 Band/Volume: NS_1_A Autor(en)/Author(s): Fricke Ronald Artikel/Article: Authorship, availability and validity of fish names described by Peter (Pehr) Simon ForssSSkål and Johann ChrisStian FabricCiusS in the ‘Descriptiones animaliumÂ’ by CarsSten Nniebuhr in 1775 (Pisces) 1-76 Stuttgarter Beiträge zur Naturkunde A, Neue Serie 1: 1–76; Stuttgart, 30.IV.2008. 1 Authorship, availability and validity of fish names described by PETER (PEHR ) SIMON FOR ss KÅL and JOHANN CHRI S TIAN FABRI C IU S in the ‘Descriptiones animalium’ by CAR S TEN NIEBUHR in 1775 (Pisces) RONALD FRI C KE Abstract The work of PETER (PEHR ) SIMON FOR ss KÅL , which has greatly influenced Mediterranean, African and Indo-Pa- cific ichthyology, has been published posthumously by CAR S TEN NIEBUHR in 1775. FOR ss KÅL left small sheets with manuscript descriptions and names of various fish taxa, which were later compiled and edited by JOHANN CHRI S TIAN FABRI C IU S . Authorship, availability and validity of the fish names published by NIEBUHR (1775a) are examined and discussed in the present paper. Several subsequent authors used FOR ss KÅL ’s fish descriptions to interpret, redescribe or rename fish species. These include BROU ss ONET (1782), BONNATERRE (1788), GMELIN (1789), WALBAUM (1792), LA C E P ÈDE (1798–1803), BLO C H & SC HNEIDER (1801), GEO ff ROY SAINT -HILAIRE (1809, 1827), CUVIER (1819), RÜ pp ELL (1828–1830, 1835–1838), CUVIER & VALEN C IENNE S (1835), BLEEKER (1862), and KLUNZIN G ER (1871). -

Acanthuroidei: Siganidae)
•».«L"WHB' vn«74MV /ir, ^/j" -w irjur- Relationships of the Fossil and Recent Genera of Rabbitfishes (Acanthuroidei: Siganidae) R • - 5Vf^> ES C. TYLt and fDREF.BAN ->: m ^ 1 •"- . *6$B O PALEO * i SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Platax Teira (Forsskål, 1775) Frequent Synonyms / Misidentifications: None / Platax Orbicularis (Non Forsskål, 1775)
click for previous page Perciformes: Acanthuroidei: Ephippidae 3619 Platax teira (Forsskål, 1775) Frequent synonyms / misidentifications: None / Platax orbicularis (non Forsskål, 1775). FAO names: En - Spotbelly batfish. 34 cm standard length 25 cm standard length Diagnostic characters: Body orbicular and strongly compressed, its depth more than twice length of head and 0.9 to 1.2 times in standard length. Head length 2.7 to 3.5 times in standard length. Large adults (above 35 cm standard length) with bony hump from top of head to interorbital region, the front head profile almost vertical; interorbital width 42 to 50% head length. Jaws with bands of slender, flattened, tricuspid teeth, the middle cusp slightly longer than lateral cusps; vomer with a few teeth, but none on palatines. Five pores on each side of lower jaw. Preopercle smooth; opercle without 20 cm 12 cm 9.4 cm spines. Dorsal fin single, with V or VI spines standard length standard length standard length and 29 to 34 soft rays, the spines hidden in front margin of fin, the last spine longest; anal fin with III spines and 21 to 26 soft rays; juveniles with pelvic fins and anterior soft rays of dorsal and anal fins elongated, but pelvic fins not reaching much past vertical at rear end of anal-fin base; pectoral fins shorter than head, with 16 to 18 rays; caudal fin truncate. Scales small and rough. Lateral line complete, with 56 to 66 scales. Colour: yellowish silvery or dusky, with a black (or dusky) bar through eye and another dark bar from dorsal-fin origin across rear edge of operculum and pectoral-fin base to belly, where it usually encloses a black blotch, with another smaller black vertical streak often present at origin of anal fin; median fins dusky yellow, with black margins posteriorly; pelvic fins yellow, dusky yellow or blackish. -

Monogenea: Capsalidae Baird, 1853: Trochopodinae) Parasite of Platax Teira, from Iraqi Marine Water, Arab Gulf Majid Abdul Aziz Bannai and Essa T
quac d A ul n tu a r e s e J i o r u e r h Bannai and Muhammad, Fish Aquac J 2015, 6:2 n s i a F l Fisheries and Aquaculture Journal DOI: 10.4172/2150-3508.1000127 ISSN: 2150-3508 ResearchResearch Article Article OpenOpen Access Access Sprostoniella teria Sp. Nov. (Monogenea: Capsalidae Baird, 1853: Trochopodinae) Parasite of Platax teira, from Iraqi Marine Water, Arab Gulf Majid Abdul Aziz Bannai and Essa T. Muhammad Aquaculture and Marine Fisheries, Marine Science Center, University of Basra, Iraq Abstract During the investigation of five species of Platax teira where collecting from Arabian Gulf. One parasite was detected Sprostoniella sp. Capsalidae Baird, 1853 from gill filaments. Results give an indication that the parasite are consider as new species in Iraqi marine and Platax teira fishes as anew host in words and new geographical distribution. Keywords: Monogenea; Sprostoniella teria; Monogenea; Capsalidae spp. (Capsalidae) including Capsala naffari n. sp. infecting mackerel Baird; Trochopodinae; Platax teira tuna Euthynnus affinis from coasts of Emirates. Three species of the genus Capsala including Capsala naffari n. sp., C. neothunni [2] and Introduction C. nozawae (Goto, 1894) are recorded and described from the buccal The Monogenea is a class of Platyhelminthes parasitic mostly cavity of mackerel tuna Euthynnus affinis caught from Emirate coasts. Capsala naffari can be differentiated by its lateral spiniform teeth, on external surfaces and gills of freshwater and marine fishes. The which extend posteriorly, small measurements compared with the Capsalidae are monogeneans parasitizing ‘skin’, fins and gills of closely resembled C. gotoi and relatively large testes. -

Fish Movement in the Red Sea and Implications for Marine Protected Area Design
Fish Movement in the Red Sea and Implications for Marine Protected Area Design Thesis by Irene Antonina Salinas Akhmadeeva In Partial Fulfillment of the Requirements For the Degree of Master of Science King Abdullah University of Science and Technology Thuwal, Kingdom of Saudi Arabia April, 2021 2 EXAMINATION COMMITTEE PAGE The thesis of Irene Antonina Salinas Akhmadeeva is approved by the examination committee. Committee Chairperson: Prof. Michael L. Berumen Committee Co-Chair: Dr. Alison Green Committee Members: Dr. Darren Coker, Prof. Rusty Brainard 3 COPYRIGHT © April 2021 Irene Antonina Salinas Akhmadeeva All Rights Reserved 4 ABSTRACT Fish Movement in the Red Sea and Implications for Marine Protected Area Design Irene Antonina Salinas Akhmadeeva The Red Sea is valued for its biodiversity and the livelihoods it provides for many. It now faces overfishing, habitat degradation, and anthropogenic induced climate-change. Marine Protected Areas (MPAs) became a powerful management tool to protect vulnerable species and ecosystems, re-establish their balance, and enhance marine populations. For this, they need to be well designed and managed. There are 15 designated MPAs in the Red Sea but their level of enforcement is unclear. To design an MPA it is necessary to know if it will protect species of interest by considering their movement needs. In this thesis I aim at understanding fish movement in the Red Sea, specifically home range (HR) to inform MPA size designation. With not much empirical data available on HR for Red Sea fish, I used a Machine Learning (ML) classification model, trained with empirical literature HR measurements with Maximum Total Length (L Max), Aspect Ratio (AR) of the caudal fin, and Trophic Level as predictor variables.