Infrared Spectrum of Bromochlorofluoromethane Earle K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cylinder Valve Selection Quick Reference for Valve Abbreviations
SHERWOOD VALVE COMPRESSED GAS PRODUCTS Appendix Cylinder Valve Selection Quick Reference for Valve Abbreviations Use the Sherwood Cylinder Valve Series Abbreviation Chart on this page with the Sherwood Cylinder Valve Selection Charts found on pages 73–80. The Sherwood Cylinder Valve Selection Chart are for reference only and list: • The most commonly used gases • The Compressed Gas Association primary outlet to be used with each gas • The Sherwood valves designated for use with this gas • The Pressure Relief Device styles that are authorized by the DOT for use with these gases PLEASE NOTE: The Sherwood Cylinder Valve Selection Charts are partial lists extracted from the CGA V-1 and S-1.1 pamphlets. They can change without notice as the CGA V-1 and S-1.1 pamphlets are amended. Sherwood will issue periodic changes to the catalog. If there is any discrepancy or question between these lists and the CGA V-1 and S-1.1 pamphlets, the CGA V-1 and S-1.1 pamphlets take precedence. Sherwood Cylinder Valve Series Abbreviation Chart Abbreviation Sherwood Valve Series AVB Small Cylinder Acetylene Wrench-Operated Valves AVBHW Small Cylinder Acetylene Handwheel-Operated Valves AVMC Small Cylinder Acetylene Wrench-Operated Valves AVMCHW Small Cylinder Acetylene Handwheel-Operated Valves AVWB Small Cylinder Acetylene Wrench-Operated Valves — WB Style BV Hi/Lo Valves with Built-in Regulator DF* Alternative Energy Valves GRPV Residual Pressure Valves GV Large Cylinder Acetylene Valves GVT** Vertical Outlet Acetylene Valves KVAB Post Medical Valves KVMB Post Medical Valves NGV Industrial and Chrome-Plated Valves YVB† Vertical Outlet Oxygen Valves 1 * DF Valves can be used with all gases; however, the outlet will always be ⁄4"–18 NPT female. -

Evolution of Refrigerants Mark O
This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. pubs.acs.org/jced Review (R)Evolution of Refrigerants Mark O. McLinden* and Marcia L. Huber Cite This: J. Chem. Eng. Data 2020, 65, 4176−4193 Read Online ACCESS Metrics & More Article Recommendations *sı Supporting Information ABSTRACT: As we enter the “fourth generation” of refrigerants, we consider the evolution of refrigerant molecules, the ever- changing constraints and regulations that have driven the need to consider new molecules, and the advancements in the tools and property models used to identify new molecules and design equipment using them. These separate aspects are intimately intertwined and have been in more-or-less continuous development since the earliest days of mechanical refrigeration, even if sometimes out-of-sight of the mainstream refrigeration industry. We highlight three separate, comprehensive searches for new refrigerantsin the 1920s, the 1980s, and the 2010sthat sometimes identified new molecules, but more often, validated alternatives already under consideration. A recurrent theme is that there is little that is truly new. Most of the “new” refrigerants, from R-12 in the 1930s to R- 1234yf in the early 2000s, were reported in the chemical literature decades before they were considered as refrigerants. The search for new refrigerants continued through the 1990s even as the hydrofluorocarbons (HFCs) were becoming the dominant refrigerants in commercial use. This included a return to several long-known natural refrigerants. Finally, we review the evolution of the NIST REFPROP database for the calculation of refrigerant properties. -

"Fluorine Compounds, Organic," In: Ullmann's Encyclopedia Of
Article No : a11_349 Fluorine Compounds, Organic GU¨ NTER SIEGEMUND, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany WERNER SCHWERTFEGER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany ANDREW FEIRING, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States BRUCE SMART, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States FRED BEHR, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States HERWARD VOGEL, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States BLAINE MCKUSICK, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States 1. Introduction....................... 444 8. Fluorinated Carboxylic Acids and 2. Production Processes ................ 445 Fluorinated Alkanesulfonic Acids ...... 470 2.1. Substitution of Hydrogen............. 445 8.1. Fluorinated Carboxylic Acids ......... 470 2.2. Halogen – Fluorine Exchange ......... 446 8.1.1. Fluorinated Acetic Acids .............. 470 2.3. Synthesis from Fluorinated Synthons ... 447 8.1.2. Long-Chain Perfluorocarboxylic Acids .... 470 2.4. Addition of Hydrogen Fluoride to 8.1.3. Fluorinated Dicarboxylic Acids ......... 472 Unsaturated Bonds ................. 447 8.1.4. Tetrafluoroethylene – Perfluorovinyl Ether 2.5. Miscellaneous Methods .............. 447 Copolymers with Carboxylic Acid Groups . 472 2.6. Purification and Analysis ............. 447 8.2. Fluorinated Alkanesulfonic Acids ...... 472 3. Fluorinated Alkanes................. 448 8.2.1. Perfluoroalkanesulfonic Acids -

Inorganic Chemistry for Dummies® Published by John Wiley & Sons, Inc
Inorganic Chemistry Inorganic Chemistry by Michael L. Matson and Alvin W. Orbaek Inorganic Chemistry For Dummies® Published by John Wiley & Sons, Inc. 111 River St. Hoboken, NJ 07030-5774 www.wiley.com Copyright © 2013 by John Wiley & Sons, Inc., Hoboken, New Jersey Published by John Wiley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada No part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning or otherwise, except as permitted under Sections 107 or 108 of the 1976 United States Copyright Act, without either the prior written permis- sion of the Publisher, or authorization through payment of the appropriate per-copy fee to the Copyright Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 646-8600. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, or online at http://www.wiley. com/go/permissions. Trademarks: Wiley, the Wiley logo, For Dummies, the Dummies Man logo, A Reference for the Rest of Us!, The Dummies Way, Dummies Daily, The Fun and Easy Way, Dummies.com, Making Everything Easier, and related trade dress are trademarks or registered trademarks of John Wiley & Sons, Inc. and/or its affiliates in the United States and other countries, and may not be used without written permission. All other trade- marks are the property of their respective owners. John Wiley & Sons, Inc., is not associated with any product or vendor mentioned in this book. -

THOMAS MIDGLEY, JR., and the INVENTION of CHLOROFLUOROCARBON REFRIGERANTS: IT AIN’T NECESSARILY SO Carmen J
66 Bull. Hist. Chem., VOLUME 31, Number 2 (2006) THOMAS MIDGLEY, JR., AND THE INVENTION OF CHLOROFLUOROCARBON REFRIGERANTS: IT AIN’T NECESSARILY SO Carmen J. Giunta, Le Moyne College The 75th anniversary of the first public Critical readers are well aware description of chlorofluorocarbon (CFC) of the importance of evaluating refrigerants was observed in 2005. A sources of information. For ex- symposium at a national meeting of ample, an article in Nature at the the American Chemical Society (ACS) end of 2005 tested the accuracy on CFCs from invention to phase-out of two encyclopedias’ entries on a (1) and an article on their invention sample of topics about science and and inventor in the Chemical Educa- history of science (3). A thought- tor (2) marked the occasion. The story ful commentary published soon of CFCs—their obscure early days as afterwards raised questions on just laboratory curiosities, their commercial what should count as an error in debut as refrigerants, their expansion into assessing such articles: omissions? other applications, and the much later disagreements among generally re- discovery of their deleterious effects on liable sources (4)? Readers of his- stratospheric ozone—is a fascinating torical narratives who are neither one of science and society well worth practicing historians nor scholarly telling. That is not the purpose of this amateurs (the category to which I article, though. aspire) may find an account of a Thomas Midgley, Jr. historical research process attrac- This paper is about contradictory Courtesy Richard P. Scharchburg tive. As a starting point, consider sources, foggy memories, the propaga- Archives, Kettering University the following thumbnail summary tion of error, and other obstacles to writ- of the invention. -

Overview of Fluorocarbons
Three Bond Technical News Issued Dec. 20, 1989 An Overview of Fluorocarbons Introduction Currently, chlorofluorocarbons are garnering a lot of Three Bond Co,. Ltd. has been working night and day in attention around the world. Chlorofluorocarbons are used in search of alternatives to chlorofluorocarbons. In the future, many of our products and when we look at the future of products that do not contain any chlorofluorocarbons will chlorofluorocarbons in the world, and in Japan, we can replace products that now contain chlorofluorocarbons. To foresee that their use will be strictly limited or eliminated all ensure these products reach our customers as smoothly as together. possible, it is important that they have a good understanding of the issues surrounding chlorofluorocarbons. Whatever the case, Three Bond Co., Ltd. is obligated to provide our customers with products that have the same functionality as chlorofluorocarbons. Contents Introduction ................................................................................................................................................................1 1. What is a fluorocarbon? .......................................................................................................................................2 2. Properties of fluorocarbons ..................................................................................................................................2 3. Types of fluorocarbons.........................................................................................................................................2 -

76 Chapt-24-Organic2
The Chemistry of Alkanes Physical Properties of Alkanes as molecular size increases so does the boiling point of the alkane increased size increased dispersion forces Alkanes Boiling Point ˚C H Methane CH 4 H C H -161.6 H H H Ethane C2H6 H C C H -88.6 H H Propane C3H8 CH3 (CH2)1 CH3 -42.1 Butane C4H10 CH3 (CH2)2 CH3 -0.5 Pentane C5H12 CH3 (CH2)3 CH3 36.1 hexane C6H14 CH3 (CH2)4 CH3 68.7 Chemical Reactions and Alkanes because the C-C and C-H bonds are relatively strong , the alkanes are fairly unreactive their inertness makes them valuable as lubricating materials and as backbone material in the construction of other hydrocarbons Combustion of Alkanes At high temperatures alkanes combust ΔH˚ CH4 + O2 CO2 + 2H2O -890.4 kJ C4H10 + 13/2O2 4CO2 + 5H2O -3119 kJ these reactions are all highly exothermic Halogenation of Alkanes at temperatures above 100 ˚C CH4 + Cl2 CH3Cl + HCl chloromethane CH3Cl + Cl2 CH2Cl2 + HCl dichloromethane CH2Cl2 + Cl2 CHCl3 + HCl trichloromethane chloroform CHCl3 + Cl2 CCl4 + HCl tetrachloromethane Carbon tetrachloride Mechanism for Halogenation of Methane CH4 + Cl2 CH3Cl + HCl hν Cl2 • Cl + • Cl hν: energy required to break the Cl-Cl bond • Cl H H C H H • Cl is very reactive and able to attack the C-H bond Mechanism for Halogenation of Methane CH4 + Cl2 CH3Cl + HCl CH4 + • Cl • CH3 + HCl H Cl H • C H H Mechanism for Halogenation of Methane CH4 + Cl2 CH3Cl + HCl • CH3 + Cl2 CH3 Cl + • Cl Cl Cl H • Cl • C H H H Cl C H H Mechanism for Halogenation of Methane Cl2 • Cl + • Cl chlorine free radical CH4 + • Cl • CH3 + HCl -
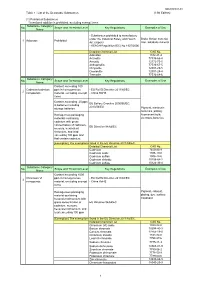
Table 1 : List of the Declarable Substances (1) Prohibited
DQ2000001-03 Table 1 : List of the Declarable Substances (13th Edition) (1) Prohibited Substances *Intentional addition is prohibited, excluding exempt items Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name - Substances prohibited to manufacture under the Industrial Safety and Health Brake friction material, 1 Asbestos Prohibited Act (Japan) filler, adiabatic material - REACH Regulation (EC) No 1907/2006 Detailed Chemical List CAS No. Asbestos 1332-21-4 Actinolite 77536-66-4 Amosite 12172-73-5 Anthophyllite 77536-67-5 Chrysotile 12001-29-5 Crocidolite 12001-28-4 Tremolite 77536-68-6 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 100 Cadmium/cadmium ppm in homogeneous - EU RoHS Directive 2011/65/EC 2 compounds material, excluding exempt - China RoHS items Content exceeding 20 ppm EU Battery Directive 2006/66/EC, in batteries including 2013/56/EU storage batteries Pigment, electronic materials, plating, Homogenous packaging fluorescent bulb, materials containing electrode,batteries cadmium with gross concentration of cadmium, EU Directive 94/62/EC mercury, hexavalent chromium, and lead exceeding 100 ppm and that contain cadmium [Exemption] The exemptions listed in the EU Directive 2011/65/EC. Detailed Chemical List CAS No. Cadmium 7440-43-9 Cadmium oxide 1306-19-0 Cadmium sulfide 1306-23-6 Cadmium chloride 10108-64-2 Cadmium sulfate 10124-36-4 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 1000 Chromium VI ppm in homogeneous - EU RoHS Directive 2011/65/EC 3 compounds material, excluding exempt - China RoHS items Homogenous packaging Pigment, catalyst, material containing plating, dye, surface hexavalent chromium with treatment gross concentration of EU Directive 94/62/EC cadmium, mercury, hexavalent chromium, and lead exceeding 100 ppm [Exemption] The exemptions listed in the EU Directive 2011/65/EC. -

Standard Thermodynamic Properties of Chemical
STANDARD THERMODYNAMIC PROPERTIES OF CHEMICAL SUBSTANCES ∆ ° –1 ∆ ° –1 ° –1 –1 –1 –1 Molecular fH /kJ mol fG /kJ mol S /J mol K Cp/J mol K formula Name Crys. Liq. Gas Crys. Liq. Gas Crys. Liq. Gas Crys. Liq. Gas Ac Actinium 0.0 406.0 366.0 56.5 188.1 27.2 20.8 Ag Silver 0.0 284.9 246.0 42.6 173.0 25.4 20.8 AgBr Silver(I) bromide -100.4 -96.9 107.1 52.4 AgBrO3 Silver(I) bromate -10.5 71.3 151.9 AgCl Silver(I) chloride -127.0 -109.8 96.3 50.8 AgClO3 Silver(I) chlorate -30.3 64.5 142.0 AgClO4 Silver(I) perchlorate -31.1 AgF Silver(I) fluoride -204.6 AgF2 Silver(II) fluoride -360.0 AgI Silver(I) iodide -61.8 -66.2 115.5 56.8 AgIO3 Silver(I) iodate -171.1 -93.7 149.4 102.9 AgNO3 Silver(I) nitrate -124.4 -33.4 140.9 93.1 Ag2 Disilver 410.0 358.8 257.1 37.0 Ag2CrO4 Silver(I) chromate -731.7 -641.8 217.6 142.3 Ag2O Silver(I) oxide -31.1 -11.2 121.3 65.9 Ag2O2 Silver(II) oxide -24.3 27.6 117.0 88.0 Ag2O3 Silver(III) oxide 33.9 121.4 100.0 Ag2O4S Silver(I) sulfate -715.9 -618.4 200.4 131.4 Ag2S Silver(I) sulfide (argentite) -32.6 -40.7 144.0 76.5 Al Aluminum 0.0 330.0 289.4 28.3 164.6 24.4 21.4 AlB3H12 Aluminum borohydride -16.3 13.0 145.0 147.0 289.1 379.2 194.6 AlBr Aluminum monobromide -4.0 -42.0 239.5 35.6 AlBr3 Aluminum tribromide -527.2 -425.1 180.2 100.6 AlCl Aluminum monochloride -47.7 -74.1 228.1 35.0 AlCl2 Aluminum dichloride -331.0 AlCl3 Aluminum trichloride -704.2 -583.2 -628.8 109.3 91.1 AlF Aluminum monofluoride -258.2 -283.7 215.0 31.9 AlF3 Aluminum trifluoride -1510.4 -1204.6 -1431.1 -1188.2 66.5 277.1 75.1 62.6 AlF4Na Sodium tetrafluoroaluminate -

“Solid,” and Stereochemistry Refers to Chemistry in Three Dimensions
CHAPTER 7 STEREOCHEMISTRY he Greek word stereos means “solid,” and stereochemistry refers to chemistry in three dimensions. The foundations of organic stereochemistry were laid by Jacobus Tvan’t Hoff* and Joseph Achille Le Bel in 1874. Independently of each other, van’t Hoff and Le Bel proposed that the four bonds to carbon were directed toward the cor- ners of a tetrahedron. One consequence of a tetrahedral arrangement of bonds to carbon is that two compounds may be different because the arrangement of their atoms in space is different. Isomers that have the same constitution but differ in the spatial arrangement of their atoms are called stereoisomers. We have already had considerable experience with certain types of stereoisomers—those involving cis and trans substitution patterns in alkenes and in cycloalkanes. Our major objectives in this chapter are to develop a feeling for molecules as three- dimensional objects and to become familiar with stereochemical principles, terms, and notation. A full understanding of organic and biological chemistry requires an awareness of the spatial requirements for interactions between molecules; this chapter provides the basis for that understanding. 7.1 MOLECULAR CHIRALITY: ENANTIOMERS Everything has a mirror image, but not all things are superposable on their mirror images. Mirror-image superposability characterizes many objects we use every day. Cups and saucers, forks and spoons, chairs and beds are all identical with their mirror images. Many other objects though—and this is the more interesting case—are not. Your left hand and your right hand, for example, are mirror images of each other but can’t be made to coin- cide point for point, palm to palm, knuckle to knuckle, in three dimensions. -

1 Three-Dimensional Structure of a Molecule
Ch 1 Three dimentional structure of a molecule 1 Three-dimensional structure of a molecule Purpose In this chapter, you will learn that it is sometimes not sufficient to describe the structure of a molecule in a planar, two-dimensional way, which is usually used in textbooks, because the atoms that make up a molecule are arranged three-dimensionally in space. The three-dimensional structures of organic compounds significantly depend on the shapes and characteristics of the hybridized atomic orbitals of the carbon atom. Here you learn about sp3, sp2 and sp hybridized orbitals, which are involved deeply in the structure of molecules. Models are useful tools to conceptualize the shapes of molecules. The commercially available molecular models will be classified based on their features. Sometimes a compound may have two or more names. Similarly, several nomenclatures are used in discussing the stereochemistry of organic compounds. The nomenclature based on the sequence rule will be introduced because this method corresponds to the systematic nomenclature that is most widely used. New terms and concepts molecular formula rational formula structural formula dihedral angle (Compare with tortion angle in Ch. 2) carbon atomic orbital sp3 hybridization tetrahedral structure configurational isomer enantiomer (enantio isomer) Configuration (steric configuration) sp2 hybridization sp hybridization molecular model space filling model skeletal model ball and stick model free rotation restricted rotation sequence rule replica atom Goals of this chapter After you master this chapter successfully, you will be able to do the following: 1to understand that organic compounds are three-dimensional, and that you cannot properly draw these on a two-dimensional sheet of paper. -

State of Illinois Environmental Protection Agency Application for Environmental Laboratory Accreditation
State of Illinois Environmental Protection Agency Application for Environmental Laboratory Accreditation Attachment 6 Program: RCRA Field of Testing: Solid and Chemical Materials, Organic Matrix: Non Solid and Potable Chemical Accredited Water Materials Analyte: Status* Method (SW846): 1,2-Dibromo-3-chloropropane (DBCP) 8011 1,2-Dibromoethane (EDB) 8011 1,4-Dioxane 8015B 1-Butanol (n-Butyl alcohol) 8015B 1-Propanol 8015B 2-Butanone (Methyl ethyl ketone, MEK) 8015B 2-Chloroacrylonitrile 8015B 2-Methyl-1-propanol (Isobutyl alcohol) 8015B 2-Methylpyridine (2-Picoline) 8015B 2-Pentanone 8015B 2-Propanol (Isopropyl alcohol) 8015B 4-Methyl-2-pentanone (Methyl isobutyl ketone, 8015B MIBK) Acetone 8015B Acetonitrile 8015B Acrolein (Propenal) 8015B Acrylonitrile 8015B Allyl alcohol 8015B Crotonaldehyde 8015B Diesel range organics (DRO) 8015B Diethyl ether 8015B Ethanol 8015B Ethyl acetate 8015B Ethylene glycol 8015B Ethylene oxide 8015B Gasoline range organics (GRO) 8015B Hexafluoro-2-methyl-2-propanol 8015B * PI: Pending Initial Accreditation A: Accredited SP: Suspended WD: Accreditation Withdrawn Attachment 6: Page 1 of 62 Program: RCRA Field of Testing: Solid and Chemical Materials, Organic Matrix: Non Solid and Potable Chemical Accredited Water Materials Analyte: Status* Method (SW846): Hexafluoro-2-propanol 8015B Isopropyl benzene ((1-methylethyl) benzene) 8015B Methanol 8015B N-Nitrosodi-n-butylamine (N-Nitrosodibutylamine) 8015B o-Toluidine 8015B Paraldehyde 8015B Propionitrile (Ethyl cyanide) 8015B Pyridine 8015B t-Butyl alcohol 8015B