Metamorphosis Vol 9(2) Complete.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Metamorphosis Issn 1018–6490 (Print) Issn 2307–5031 (Online) Lepidopterists’ Society of Africa
Volume 31: 4–6 METAMORPHOSIS ISSN 1018–6490 (PRINT) ISSN 2307–5031 (ONLINE) LEPIDOPTERISTS’ SOCIETY OF AFRICA NOTE Unique genitalic structure in a West African lycaenid butterfly, Liptena seyboui Warren-Gash & Larsen, 2003 (Lepidoptera, Lycaenidae, Poritiinae, Liptenini) Published online: 26 February 2020 Szabolcs Sáfián1 & Jadwiga Lorenc-Brudecka2 1 African Natural History Research Trust, Street Court, Kingsland, Leominster, Herefordshire, HR6 9QA, UK. E-mail: [email protected] 2 Nature Education Centre, Jagiellonian University, Gronostajowa 5, 30-387 Kraków, Poland. E-mail: [email protected] Copyright © Lepidopterists’ Society of Africa INTRODUCTION possible to produce during the time of description. The Liberian specimen is also illustrated (Fig. 2). Liptena Westwood, [1851] is a large, quite heterogeneic genus distributed solely in the Afrotropical region with the Specimen data: ♂ LIBERIA, Wologizi Mountains, Ridge majority of species being restricted to the main Guineo- Camp 2, 8°7'20.79"N, 9°56'50.75"W, 883 m, 22– 31.xi.2018. General collecting. Sáfián, Sz., Simonics, G. Congolian forest zone and only a few occurring in the Leg. ANHRT: 2018.43. ANHRT unique number: southern (Zambezian) and northern (Guinea savannah) ANHRTUK00058074. transition zone and dense woodland, savannah area (Larsen 1991, 2005). Stempffer’s (1967) terminology of genitalia characters are used to described the genitalic features of L. seyboui with Male genitalia of Liptena are discussed extensively by slight modifications, where no appropriate association Stempffer (1967) and Stempffer et al. (1974), who also was possible. Genitalia were dissected using KOH illustrated genitalia of at least one species of each defined solution to dissolve soft abdominal tissue. -
The Butterflies of Kenya and Uganda, Part 5
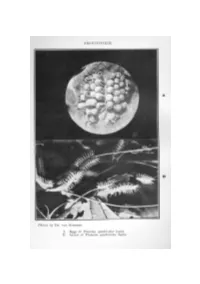
FRONTISPIECE. A B Photos by DR. VAN SOMEREN. A. Eggs of Planema quadricolor leptis. B. Larval of Planema quadricolor leptis. THE BUTTERFLIES OF KENYA AND UGANDA. By V. G. L. VANSOMEBEN,F.E.S., F.L.S., etc., and REV. K. ST. A. ROGERS,M.A., F.E.S. PARTV. SUB-FAMILYAORiEINiE. GENUSPLANEMA, Dbld. & Hew. INTRODUCTION. Representatives of the genus Planema are to be distinguished from Acuea, by the colour of the Palps, which are black with a lateral grey or white line, and by the position of the first branch of the subcostal or 11th vein; this is given off, at or beyond the apex of the cell. The hind-wing cell is usually much smaller than in ACTma. The ~arvre of the two genera are not markedly different, but those of Planema usually have much longer branched spines and on the whole I1relessparticoloured. The pupre are distinguishable how• ever; those of Planema always have long dorsal spines which are hooked at the end, but the body of the pupa is not heavily ornamented with dark markings. Most of the repre.sentatives of this genus are conspicuously coloured, many having a general superficial resemblance to each other. All are credited with a high degree of distastefulness to enemies, and as a reE\ult quite a number /l.re PUP.IE OF PLANEMA mimicked by species of other genera and families. Some of the most wonderful examples of mimicry are to be found between species of Planema and the Nymphalid8, P8euda()1la3a; exampleE\ are given later. 29 PLANEMA POGGEI NELSON!, Smth. -

Title Lorem Ipsum Dolor Sit Amet, Consectetur Adipiscing Elit
Volume 26: 102–108 METAMORPHOSIS www.metamorphosis.org.za ISSN 1018–6490 (PRINT) LEPIDOPTERISTS’ SOCIETY OF AFRICA ISSN 2307–5031 (ONLINE) Classification of the Afrotropical butterflies to generic level Published online: 25 December 2015 Mark C. Williams 183 van der Merwe Street, Rietondale, Pretoria, South Africa. E-mail: [email protected] Copyright © Lepidopterists’ Society of Africa Abstract: This paper applies the findings of phylogenetic studies on butterflies (Papilionoidea) in order to present an up to date classification of the Afrotropical butterflies to genus level. The classification for Afrotropical butterflies is placed within a worldwide context to subtribal level. Taxa that still require interrogation are highlighted. Hopefully this classification will provide a stable context for researchers working on Afrotropical butterflies. Key words: Lepidoptera, Papilionoidea, Afrotropical butterflies, classification. Citation: Williams, M.C. (2015). Classification of the Afrotropical butterflies to generic level. Metamorphosis 26: 102–108. INTRODUCTION Suborder Glossata Fabricius, 1775 (6 infraorders) Infraorder Heteroneura Tillyard, 1918 (34 Natural classifications of biological organisms, based superfamilies) on robust phylogenetic hypotheses, are needed before Clade Obtectomera Minet, 1986 (12 superfamilies) meaningful studies can be conducted in regard to their Superfamily Papilionoidea Latreille, 1802 (7 evolution, biogeography, ecology and conservation. families) Classifications, dating from the time of Linnaeus in the Family Papilionidae Latreille, 1802 (32 genera, 570 mid seventeen hundreds, were based on morphology species) for nearly two hundred and fifty years. Classifications Family Hedylidae Guenée, 1858 (1 genus, 36 species) based on phylogenies derived from an interrogation of Family Hesperiidae Latreille, 1809 (570 genera, 4113 the genome of individual organisms began in the late species) 20th century. -

The Genus Acraea (Lepidoptera : Nymphalidae) - Peter Hendry
The genus Acraea (Lepidoptera : Nymphalidae) - Peter Hendry With the recent migration to Australia of the Tawny Coster (Acraea terpsicore (Linnaeus, 1758)), (see Creature Feature this issue), I thought it might be timely to take a look at the genus worldwide. It must be noted that due to a misidentification A. terpsicore had long been known as A. violae and many references in the literature and on the web refer to it as A. violae. As with much of the Lepidoptera the genus is in a state of flux, and has long been split into the subgenera Acraea (Acraea) and Acraea (Actinote). The genus is placed in the tribe Acraeini and until Harvey (1991) placed it in the subfamily Heliconiinae it was listed in the subfamily Acraeinae. Recent molecular work has made changes and a current listing of the tribe Acraeini, by Niklas Wahlberg, is available at http://www.nymphalidae.net/Classification/Acraeini.htm. It shows members of the old subgenus Acraea (Actinote) being placed in the genus Actinote, and the old subgenus Acraea (Acraea) becoming the genus Acraea with a subgenus Acraea (Bematistes). It also lists several Acraea as unplaced. This may further change as some believe the subgenus Acraea (Bematistes) will move to the genus Bematistes. The genus is primarily Afrotropical with only four species occurring outside this region, these being, Acraea andromacha (Fig. 1) A. meyeri (Fig. 10) A. moluccana and A. terpsicore. A fifth species the Yellow Coster Acraea (Actinote) issoria is now referred to the genus Actinote. Like many of the Nymphalidae the larvae feed on plants which contain cyanogens making the larvae and adults poisonous to predators. -

African Butterfly News!
LATE WINTER EDITION: JULY / AUGUST AFRICAN 2017-4 THE BUTTERFLY LEPIDOPTERISTS’ SOCIETY OF AFRICA NEWS LATEST NEWS Welcome to the Late Winter edition of African Butterfly News! African Butterfly News celebrates its first year of existence; the first edition, 2016-5, was circulated in September last year. The photographic competition commenced in August 2016, so the annual award will be made in the next, Spring Edition – for purposes of the photographic competition, the season starts in August and ends in July. A reminder that the newsletter is circulated every two months: Late Summer (January and February) – circulated in January Autumn (March and April) – circulated in March Early Winter (May and June) – circulated in May Late Winter (July and August) – circulated in July Spring (September and October) – circulated in September Early Summer (November and December) – circulated in November You will all be aware of the devastating fires that the southern Cape experienced in June. Dave and Hanna Edge, LepSoc Africa’s Treasurer and Membership Secretary respectively, were caught up in this drama, and had to evacuate their house (refer to Dave’s eyewitness account below). Fortunately, the building survived the fire, although the nearby Brenton Blue Reserve was incinerated. All fences, sign boards and marker-posts were destroyed – it is hoped that some larvae or pupae of the Brenton Blue (Orachrysops niobe) are still alive, safely underground. See a report under COREL under the “Projects” section. Some of you may have seen an article in the Sunday Times, by Aaron Hyman, a friend of Christopher Dobson. This magazine relies on material from you, the members of LepSoc Africa. -

Check-List of the Butterflies of the Kakamega Forest Nature Reserve in Western Kenya (Lepidoptera: Hesperioidea, Papilionoidea)
Nachr. entomol. Ver. Apollo, N. F. 25 (4): 161–174 (2004) 161 Check-list of the butterflies of the Kakamega Forest Nature Reserve in western Kenya (Lepidoptera: Hesperioidea, Papilionoidea) Lars Kühne, Steve C. Collins and Wanja Kinuthia1 Lars Kühne, Museum für Naturkunde der Humboldt-Universität zu Berlin, Invalidenstraße 43, D-10115 Berlin, Germany; email: [email protected] Steve C. Collins, African Butterfly Research Institute, P.O. Box 14308, Nairobi, Kenya Dr. Wanja Kinuthia, Department of Invertebrate Zoology, National Museums of Kenya, P.O. Box 40658, Nairobi, Kenya Abstract: All species of butterflies recorded from the Kaka- list it was clear that thorough investigation of scientific mega Forest N.R. in western Kenya are listed for the first collections can produce a very sound list of the occur- time. The check-list is based mainly on the collection of ring species in a relatively short time. The information A.B.R.I. (African Butterfly Research Institute, Nairobi). Furthermore records from the collection of the National density is frequently underestimated and collection data Museum of Kenya (Nairobi), the BIOTA-project and from offers a description of species diversity within a local literature were included in this list. In total 491 species or area, in particular with reference to rapid measurement 55 % of approximately 900 Kenyan species could be veri- of biodiversity (Trueman & Cranston 1997, Danks 1998, fied for the area. 31 species were not recorded before from Trojan 2000). Kenyan territory, 9 of them were described as new since the appearance of the book by Larsen (1996). The kind of list being produced here represents an information source for the total species diversity of the Checkliste der Tagfalter des Kakamega-Waldschutzge- Kakamega forest. -

Download Full-Text
Research in Zoology 2015, 5(2): 32-37 DOI: 10.5923/j.zoology.20150502.02 First Records of Butterfly Diversity on Two Remote Islands on the Volta Lake of Ghana, the Largest Reservoir by Total Surface Area in the World Daniel Opoku Agyemang1, Daniel Acquah-Lamptey1,*, Roger Sigismond Anderson2, Rosina Kyerematen1,2 1Department of Animal Biology and Conservation Science, University of Ghana, Legon, Ghana 2African Regional Postgraduate Programme in Insect Science, University of Ghana, Legon, Ghana Abstract The construction of the Akosombo Dam in Ghana for hydroelectric energy led to the creation of many islands on the Volta Lake. The biological diversity on these islands is unknown and so a rapid assessment was conducted in January 2014 as part as a region wide assessment to determine the butterfly diversity on two of these islands, Biobio and Agbasiagba. Diversity indices were computed for both islands using the Shannon-Weiner index, Margalef’s index for richness and Whittaker’s index for comparison of diversity between the two islands. A total of eight hundred and eighty-one (881) individual butterflies representing forty-five (45) species belonging to eight (8) families were recorded during the study. Thirty-nine (39) species of butterflies were recorded on Biobio island whiles twenty-eight (28) species were recorded on Agbasiagba. This was expected as the larger islands are expected to support more species than smaller ones, with Biobio island being relatively bigger than Agbasiagba. The shared species of butterflies on both islands were twenty-two (22) representing 48.9% of the total species accumulated. Indicator species like Junonia oenone, Danaus chrysippus and Papilio demodocus were also recorded indicating the degraded floral quality of the Islands. -

304 Genus Eresiomera Clench
AFROTROPICAL BUTTERFLIES 17th edition (2018). MARK C. WILLIAMS. http://www.lepsocafrica.org/?p=publications&s=atb Genus Eresiomera Clench, 1965 In Fox et al., 1965. Memoirs of the American Entomological Society No. 19: 290 (438 pp.). Type-species: Liptena isca Hewitson, by original designation. The genus Eresiomera belongs to the Family Lycaenidae Leach, 1815; Subfamily Poritiini Doherty, 1886; Tribe Liptenini Röber, 1892. The other genera in the Tribe Liptenini in the Afrotropical Region are Liptena, Obania, Kakumia, Tetrarhanis, Falcuna, Larinopoda, Micropentila, Pseuderesia, Eresina, Parasiomera, Citrinophila, Argyrocheila, Teriomima, Euthecta, Baliochila, Cnodontes, Congdonia, Eresinopsides, Toxochitona, Mimacraea and Mimeresia. Eresiomera (Pearlys) is a purely Afrotropical genus containing 17 species. Most species have a weak flight, usually slowly circling tree trunks high up, in the vicinity of Crematogaster ant nests (Larsen, 2005a). *Eresiomera bicolor (Grose-Smith & Kirby, [1890]) Western Pearly Pseuderesia bicolor Grose-Smith & Kirby, [1890]. In Grose-Smith & Kirby, [1887-92]. Rhopalocera exotica, being illustrations of new, rare and unfigured species of butterflies 1: 44 (183 pp.). London. Eresiomera bicolor Grose-Smith & Kirby, 1890. d’Abrera, 2009: 634. Eresiomera bicolor. Male. Left – upperside; right – underside. Bobiri Forest, Ghana. 23 May 2014. Images M.C.Williams ex Gardiner Collection. Eresiomera bicolor. Female. Left – upperside; right – underside. Bobiri Forest, Ghana. 24 May 2014. Images M.C.Williams ex Gardiner Collection. 1 Type locality: “Accu”. [= Accra? (Larsen, 2005a)]. Distribution: Sierra Leone, Liberia, Ivory Coast, Ghana, Togo, Nigeria. Specific localities: Ghana – ?Accra (TL); Bobiri Butterfly Sanctuary (Larsen et al., 2007); Boabeng-Fiema Monkey Sanctuary (Larsen et al., 2009). Habitat: Forest. Habits: A relatively common species, usually found in ones or twos (Larsen, 2005a). -

Catalogue of the Type Specimens of Lepidoptera Rhopalocera in the Hill Museum
Original from and digitized by National University of Singapore Libraries Original from and digitized by National University of Singapore Libraries Original from and digitized by National University of Singapore Libraries Original from and digitized by National University of Singapore Libraries CATALOGUE OF THE Type Specimens of Lepidoptera Rhopalocera IN THE HILL MUSEUM BY A. G. GABRIEL, F.E.S. Issued June, 1932 LONDON JOHN BALE, SONS & DANIELSSON, LTD. 83-91, GBEAT TITCHFIELD STEEET, OXEOED STEEET, W. 1 1932 Price 20/- Original from and digitized by National University of Singapore Libraries Unfortunately Mr. Joicey did not live to see the publication of this Catalogue. It will however remain, together with the four completed volumes of the " Bulletin of the Hill Museum," as a lasting memorial to to the magnificent collection of Lepidoptera amassed by Mr. Joicey, and to the work carried out at the Hill Museum under his auspices. G. Talbot. Original from and digitized by National University of Singapore Libraries CATALOGUE OF THE TYPE SPECIMENS OF LEPIDOPTERA RHOPALOCERA IN THE HILL MUSEUM. By A. G. GABRIEL, F.E.S. INTRODUCTION BY G. TALBOT. It is important to know exactly where type specimens are to be found. The British Museum set an example by publishing catalogues of some of their Rhopalocera types, and we hope this will be continued. Mr. Gabriel, who was responsible for that work, has been asked by Mr. Joicey to prepare a catalogue for the Hill Museum. The original description of almost every name in this catalogue has been examined for the correct reference, and where the sex or habitat was wrongly quoted, the necessary correction has been made. -

AGIDE Final Report
COMPTE RENDU FINAL D’EXECUTION DE PROJET I. INFORMATIONS DE BASE Nom de l’organisation : Association pour la Gestion Intégrée et Durable de l'Environnement (AGIDE) Adresses Siège social : Tsévié, Préfecture de Zio, Région maritime, TOGO B.P. 149 Tsévié – TOGO Cel. :(00228) 909 05 84 E-mail : [email protected] Antennes : Kpalimé, Préfecture de Kloto, Région des plateaux E-mail : [email protected] Titre du projet : Inventory of Butterflies in the Missahoe Classified Forest in Togo, Upper Guinea Forest II. REMARQUES PRÉALABLES 1 – Présentation sommaire du Togo Situé dans la sous région Ouest africaine, le Togo est un petit pays effilé coincé entre le Bénin à l’Est et le Ghana à l’Ouest. Il est limité au Nord par le Burkina Faso et au Sud par le Golfe de Guinée. Sa superficie est de 56 600 km2. La population est de 4 500 000 habitants avec une densité moyenne de 25 habitants / Km2. La proportion de la femme est de 62%. La zone guinéenne du Togo qui comprend les régions Maritimes et des Plateaux compte 76,6% de pauvre dont 65,5% extrêmement pauvre1. Sur le plan économique, l’évolution du PIB par habitant du Togo en général a progressivement baissé depuis les années 1997 à la suite de la situation socio politique du pays, jointe aux problèmes climatiques qui ont eu des impacts négatifs sur la flore, la faune et la production agricole2. En vue de freiner la pression anthropique sur les ressources naturelles et réduire la pauvreté des populations tributaires des ressources animales et végétales, les divers programme de développement3 proposent dans leur plan d’action, le développement des activités génératrices de revenus afin d’orienter les activités de ces exploitants. -

Mt Mabu, Mozambique: Biodiversity and Conservation
Darwin Initiative Award 15/036: Monitoring and Managing Biodiversity Loss in South-East Africa's Montane Ecosystems MT MABU, MOZAMBIQUE: BIODIVERSITY AND CONSERVATION November 2012 Jonathan Timberlake, Julian Bayliss, Françoise Dowsett-Lemaire, Colin Congdon, Bill Branch, Steve Collins, Michael Curran, Robert J. Dowsett, Lincoln Fishpool, Jorge Francisco, Tim Harris, Mirjam Kopp & Camila de Sousa ABRI african butterfly research in Forestry Research Institute of Malawi Biodiversity of Mt Mabu, Mozambique, page 2 Front cover: Main camp in lower forest area on Mt Mabu (JB). Frontispiece: View over Mabu forest to north (TT, top); Hermenegildo Matimele plant collecting (TT, middle L); view of Mt Mabu from abandoned tea estate (JT, middle R); butterflies (Lachnoptera ayresii) mating (JB, bottom L); Atheris mabuensis (JB, bottom R). Photo credits: JB – Julian Bayliss CS ‒ Camila de Sousa JT – Jonathan Timberlake TT – Tom Timberlake TH – Tim Harris Suggested citation: Timberlake, J.R., Bayliss, J., Dowsett-Lemaire, F., Congdon, C., Branch, W.R., Collins, S., Curran, M., Dowsett, R.J., Fishpool, L., Francisco, J., Harris, T., Kopp, M. & de Sousa, C. (2012). Mt Mabu, Mozambique: Biodiversity and Conservation. Report produced under the Darwin Initiative Award 15/036. Royal Botanic Gardens, Kew, London. 94 pp. Biodiversity of Mt Mabu, Mozambique, page 3 LIST OF CONTENTS List of Contents .......................................................................................................................... 3 List of Tables ............................................................................................................................. -

Transactions of the Entomological Society of London
— ( 309 ) XIX. Entomological Observations and Captures during the visit of the British Association to South Africa in 1905. By F. A. Dixey, M.A., M.D., F.E.S., and G. B. LoNGSTAFF, M.A., M.D., F.R.GP., F.E.S. [Read June 5th, 1907.] Plate XXV. Cape Town. Lat. 34° S. Sea level. August 8tli, 1905. Surely no one who was on deck when the " Kildonan Castle " anchored in Table Bay will forget the impressive scene. Behind the town-lights which gleamed along the front the grand mass of Table Mountain, clear cut against a streak of dawn, lay under the Southern Cross and Magellanic Clouds, while in the opposite quarter Jupiter and Venus shone brilliant beyond our experience, the latter reflected in the sea, and Orion standing on his head demonstrated that we were indeed in a Southern land. These astronomical facts had a bearing on our entomological operations, since we had to grow accus- tomed to the fact that the most promising hunting-grounds were slopes with a north-cast aspect. Faithful to our own science rather than to the associa- tion of which we were members, we had decided to go on to Durban by the same steamer, and put in as many days collecting as possible on the Natal Coast. This left us but a day and a half at Cape Town, in which to get a glimpse of its fauna and flora, and we were truly fortunate in that the Southern spring smiled upon us and provided, if indeed few insects, at any rate what Mr.