Molecular Characterization of Neurotransmitter Transporters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inhibitory Role for GABA in Autoimmune Inflammation
Inhibitory role for GABA in autoimmune inflammation Roopa Bhata,1, Robert Axtella, Ananya Mitrab, Melissa Mirandaa, Christopher Locka, Richard W. Tsienb, and Lawrence Steinmana aDepartment of Neurology and Neurological Sciences and bDepartment of Molecular and Cellular Physiology, Beckman Center for Molecular Medicine, Stanford University, Stanford, CA 94305 Contributed by Richard W. Tsien, December 31, 2009 (sent for review November 30, 2009) GABA, the principal inhibitory neurotransmitter in the adult brain, serum (13). Because actions of exogenous GABA on inflammation has a parallel inhibitory role in the immune system. We demon- and of endogenous GABA on phasic synaptic inhibition both strate that immune cells synthesize GABA and have the machinery occur at millimolar concentrations (5, 8, 9), we hypothesized that for GABA catabolism. Antigen-presenting cells (APCs) express local mechanisms may also operate in the peripheral immune functional GABA receptors and respond electrophysiologically to system to enhance GABA levels near the inflammatory focus. We GABA. Thus, the immune system harbors all of the necessary first asked whether immune cells have synthetic machinery to constituents for GABA signaling, and GABA itself may function as produce GABA by Western blotting for GAD, the principal syn- a paracrine or autocrine factor. These observations led us to ask thetic enzyme. We found significant amounts of a 65-kDa subtype fl further whether manipulation of the GABA pathway in uences an of GAD (GAD-65) in dendritic cells (DCs) and lower levels in animal model of multiple sclerosis, experimental autoimmune macrophages (Fig. 1A). GAD-65 increased when these cells were encephalomyelitis (EAE). Increasing GABAergic activity amelio- stimulated (Fig. -

The Concise Guide to Pharmacology 2019/20
Edinburgh Research Explorer THE CONCISE GUIDE TO PHARMACOLOGY 2019/20 Citation for published version: Cgtp Collaborators 2019, 'THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters', British Journal of Pharmacology, vol. 176 Suppl 1, pp. S397-S493. https://doi.org/10.1111/bph.14753 Digital Object Identifier (DOI): 10.1111/bph.14753 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: British Journal of Pharmacology General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 28. Sep. 2021 S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology (2019) 176, S397–S493 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters Stephen PH Alexander1 , Eamonn Kelly2, Alistair Mathie3 ,JohnAPeters4 , Emma L Veale3 , Jane F Armstrong5 , Elena Faccenda5 ,SimonDHarding5 ,AdamJPawson5 , Joanna L -

Interplay Between Metformin and Serotonin Transport in the Gastrointestinal Tract: a Novel Mechanism for the Intestinal Absorption and Adverse Effects of Metformin
INTERPLAY BETWEEN METFORMIN AND SEROTONIN TRANSPORT IN THE GASTROINTESTINAL TRACT: A NOVEL MECHANISM FOR THE INTESTINAL ABSORPTION AND ADVERSE EFFECTS OF METFORMIN Tianxiang Han A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Eshelman School of Pharmacy. Chapel Hill 2013 Approved By: Dhiren R. Thakker, Ph.D. Michael Jay, Ph.D. Kim L. R. Brouwer, Pharm.D., Ph.D. Joseph W. Polli, Ph.D. Xiao Xiao, Ph.D. © 2013 Tianxiang Han ALL RIGHTS RESERVED ii ABSTRACT TIANXIANG HAN: Interplay between Metformin and Serotonin Transport in the Gastrointestinal Tract: A Novel Mechanism for the Intestinal Absorption and Adverse Effects of Metformin (Under the direction of Dhiren R. Thakker, Ph.D.) Metformin is a widely prescribed drug for Type II diabetes mellitus. Previous studies have shown that this highly hydrophilic and charged compound traverses predominantly paracellularly across the Caco-2 cell monolayer, a well-established model for human intestinal epithelium. However, oral bioavailability of metformin is significantly higher than that of the paracellular probe, mannitol (~60% vs ~16%). Based on these observations, the Thakker laboratory proposed a “sponge” hypothesis (Proctor et al., 2008) which states that the functional synergy between apical (AP) transporters and paracellular transport enhances the intestinal absorption of metformin. This dissertation work aims to identify AP uptake transporters of metformin, determine their polarized localization, and elucidate their roles in the intestinal absorption and adverse effects of metformin. Chemical inhibition and transporter-knockdown studies revealed that four transporters, namely, organic cation transporter 1 (OCT1), plasma membrane monoamine transporter (PMAT), serotonin reuptake transporter (SERT) and choline high-affinity transporter (CHT) contribute to AP uptake of metformin in Caco-2 cells. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Tyrosine 140 of the γ-Aminobutyric Acid Transporter GAT-1 Plays A
University of Montana ScholarWorks at University of Montana Biomedical and Pharmaceutical Sciences Faculty Biomedical and Pharmaceutical Sciences Publications 1997 Tyrosine 140 of the γ-Aminobutyric Acid Transporter GAT-1 Plays a Critical Role in Neurotransmitter Recognition Yona Bismuth Michael Kavanaugh University of Montana - Missoula Baruch I. Kanner Let us know how access to this document benefits ouy . Follow this and additional works at: https://scholarworks.umt.edu/biopharm_pubs Part of the Medical Sciences Commons, and the Pharmacy and Pharmaceutical Sciences Commons Recommended Citation Bismuth, Yona; Kavanaugh, Michael; and Kanner, Baruch I., "Tyrosine 140 of the γ-Aminobutyric Acid Transporter GAT-1 Plays a Critical Role in Neurotransmitter Recognition" (1997). Biomedical and Pharmaceutical Sciences Faculty Publications. 58. https://scholarworks.umt.edu/biopharm_pubs/58 This Article is brought to you for free and open access by the Biomedical and Pharmaceutical Sciences at ScholarWorks at University of Montana. It has been accepted for inclusion in Biomedical and Pharmaceutical Sciences Faculty Publications by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 272, No. 26, Issue of June 27, pp. 16096–16102, 1997 © 1997 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Tyrosine 140 of the g-Aminobutyric Acid Transporter GAT-1 Plays a Critical Role in Neurotransmitter Recognition* (Received for publication, February 6, 1997, and in revised form, April 8, 1997) Yona Bismuth‡, Michael P. Kavanaugh§, and Baruch I. Kanner‡¶ From the ‡Department of Biochemistry, Hadassah Medical School, the Hebrew University, Jerusalem 91120, Israel and the §Vollum Institute, Oregon Health Science University, Portland, Oregon 97201 The g-aminobutyric acid (GABA) transporter GAT-1 is tify amino acid residues of GAT-1 involved in substrate bind- located in nerve terminals and catalyzes the electro- ing. -

A Review of Glutamate Receptors I: Current Understanding of Their Biology
J Toxicol Pathol 2008; 21: 25–51 Review A Review of Glutamate Receptors I: Current Understanding of Their Biology Colin G. Rousseaux1 1Department of Pathology and Laboratory Medicine, Faculty of Medicine, University of Ottawa, Ottawa, Ontario, Canada Abstract: Seventy years ago it was discovered that glutamate is abundant in the brain and that it plays a central role in brain metabolism. However, it took the scientific community a long time to realize that glutamate also acts as a neurotransmitter. Glutamate is an amino acid and brain tissue contains as much as 5 – 15 mM glutamate per kg depending on the region, which is more than of any other amino acid. The main motivation for the ongoing research on glutamate is due to the role of glutamate in the signal transduction in the nervous systems of apparently all complex living organisms, including man. Glutamate is considered to be the major mediator of excitatory signals in the mammalian central nervous system and is involved in most aspects of normal brain function including cognition, memory and learning. In this review, the basic biology of the excitatory amino acids glutamate, glutamate receptors, GABA, and glycine will first be explored. In the second part of this review, the known pathophysiology and pathology will be described. (J Toxicol Pathol 2008; 21: 25–51) Key words: glutamate, glycine, GABA, glutamate receptors, ionotropic, metabotropic, NMDA, AMPA, review Introduction and Overview glycine), peptides (vasopressin, somatostatin, neurotensin, etc.), and monoamines (norepinephrine, dopamine and In the first decades of the 20th century, research into the serotonin) plus acetylcholine. chemical mediation of the “autonomous” (autonomic) Glutamatergic synaptic transmission in the mammalian nervous system (ANS) was an area that received much central nervous system (CNS) was slowly established over a research activity. -

Prokaryotic Solute/Sodium Symporters: Versatile Functions and Mechanisms of a Transporter Family †
Review Prokaryotic Solute/Sodium Symporters: Versatile Functions and Mechanisms of a Transporter Family † Tania Henriquez, Larissa Wirtz, Dan Su and Heinrich Jung * Microbiology, Department Biology 1, Ludwig Maximilians University Munich, D-82152 Martinsried, Germany; [email protected] (T.H.); [email protected] (L.W.); [email protected] (D.S.) * Correspondence: [email protected]; Tel.: +49-89-218074630 † This article is dedicated to the memory of Ron Kaback, an exceptional scientist and great mentor. Abstract: The solute/sodium symporter family (SSS family; TC 2.A.21; SLC5) consists of integral membrane proteins that use an existing sodium gradient to drive the uphill transport of various solutes, such as sugars, amino acids, vitamins, or ions across the membrane. This large family has representatives in all three kingdoms of life. The human sodium/iodide symporter (NIS) and the sodium/glucose transporter (SGLT1) are involved in diseases such as iodide transport defect or glu- cose-galactose malabsorption. Moreover, the bacterial sodium/proline symporter PutP and the so- dium/sialic acid symporter SiaT play important roles in bacteria–host interactions. This review fo- cuses on the physiological significance and structural and functional features of prokaryotic mem- bers of the SSS family. Special emphasis will be given to the roles and properties of proteins con- taining an SSS family domain fused to domains typically found in bacterial sensor kinases. Citation: Henriquez, T.; Wirtz, L.; Keywords: secondary transport; solute/sodium symport; SLC5; PutP; signal transduction; bacterial Su, D.; Jung, H. Prokaryotic two-component systems; bacterial sensor kinase Solute/Sodium Symporters: Versatile Functions and Mechanisms of a Transporter Family. -

GAT-1, a High-Affinity GABA Plasma Membrane Transporter, Localized to Neurons and Astroglia in the Cerebral Cortex
The Journal of Neuroscience, November 1995, 75(11): 7734-7746 GAT-1, a High-Affinity GABA Plasma Membrane Transporter, Localized to Neurons and Astroglia in the Cerebral Cortex Andrea Minelli,’ Nicholas C. Brecha, 2,3,4,5,6Christine Karschiq7 Silvia DeBiasi,8 and Fiorenzo Conti’ ‘Institute of Human Physiology, University of Ancona, Ancona, Italy, *Department of Neurobiology, 3Department of Medicine, 4Brain Research Institute, and 5CURE:VA/UCLA Gastroenteric Biology Center, UCLA School of Medicine, and 6Veterans Administration Medical Center, Los Angeles, CA, 7Max-Planck-lnstitute for Experimental Medicine, Gottingen, Germany, and 8Department of General Physiology and Biochemistry, Section of Histology and Human Anatomy, University of Milan, Milan, Italy High affinity, GABA plasma membrane transporters influ- into GABAergic axon terminals, GAT-1 influences both ex- ence the action of GABA, the main inhibitory neurotrans- citatory and inhibitory transmission by modulating the mitter. The cellular expression of GAT-1, a prominent “paracrine” spread of GABA (Isaacson et al., 1993), and GABA transporter, has been investigated in the cerebral suggest that astrocytes may play an important role in this cortex of adult rats using in situ hybridizaton with %-la- process. beled RNA probes and immunocytochemistry with affinity [Key words: GABA, GABA transporlers, neocottex, syn- purified polyclonal antibodies directed to the C-terminus of apses, neurons, astrocytes] rat GAT-1. GAT-1 mRNA was observed in numerous neurons and in Synaptic transmissionmediated by GABA plays a key role in some glial cells. Double-labeling experiments were per- controlling neuronal activity and information processingin the formed to compare the pattern of GAT-1 mRNA containing mammaliancerebral cortex (Krnjevic, 1984; Sillito, 1984; Mc- and GAD67 immunoreactive cells. -

Sorting of the Vesicular GABA Transporter to Functional Vesicle Pools by an Atypical Dileucine-Like Motif
10634 • The Journal of Neuroscience, June 26, 2013 • 33(26):10634–10646 Cellular/Molecular Sorting of the Vesicular GABA Transporter to Functional Vesicle Pools by an Atypical Dileucine-like Motif Magda S. Santos,1 C. Kevin Park,1 Sarah M. Foss,1,2 Haiyan Li,1 and Susan M. Voglmaier1 1Department of Psychiatry, and 2Graduate Program in Cell Biology, University of California, San Francisco, School of Medicine, San Francisco, California 94143-0984 Increasing evidence indicates that individual synaptic vesicle proteins may use different signals, endocytic adaptors, and trafficking pathways for sorting to distinct pools of synaptic vesicles. Here, we report the identification of a unique amino acid motif in the vesicular GABA transporter (VGAT) that controls its synaptic localization and activity-dependent recycling. Mutational analysis of this atypical dileucine-like motif in rat VGAT indicates that the transporter recycles by interacting with the clathrin adaptor protein AP-2. However, mutation of a single acidic residue upstream of the dileucine-like motif leads to a shift to an AP-3-dependent trafficking pathway that preferentially targets the transporter to the readily releasable and recycling pool of vesicles. Real-time imaging with a VGAT-pHluorin fusion provides a useful approach to explore how unique sorting sequences target individual proteins to synaptic vesicles with distinct functional properties. Introduction ery to different vesicle pools, or molecular heterogeneity of SVs How proteins are sorted to synaptic vesicles (SVs) has been a that could determine their functional characteristics (Mor- long-standing question in cell biology. At the nerve terminal, genthaler et al., 2003; Salazar et al., 2004; Voglmaier and Ed- synaptic vesicles undergo exocytosis and then reform through wards, 2007; Hua et al., 2011a; Lavoie et al., 2011; Raingo et al., endocytic events. -
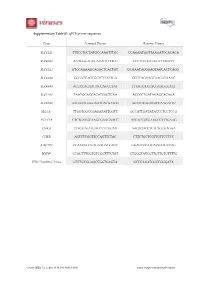
Gene Forward Primer Reverse Primer
Supplementary Table S1. qPCR primer sequences. Gene Forward Primer Reverse Primer SLC44A1 TTTCCTGCTATGCCAAGTTTGC CCAGAATGGTTAAGATCCACACA SLC44A2 AAAGGGAGGGAGAGTTTTGC CCCTTGGGTGGGTTTAGTTT SLC44A3 GTCCAAAAGCAGACTCACTGT GCAAATAGGGAGTAGCACTCAGG SLC44A4 GGGATCAGCGGTCTTATTGA GCGCAGAAGCAAGATAAAC SLC44A5 ACCCCAGAAGAGCAGCCTAT TTTAGCAACACGGAGGGACT SLC22A1 TAATGGACCACATCGCTCAA ACCCCTGATAGAGCACAGA SLC22A2 AAGAATGGGGAATCACAATGG AGATGTGGACGCCAAGATTC SLC5A7 TTGGTGGCCGAGATATTGGTT GCCATTGATATACCCTCCTCCG PCYT1A CTCTGATGCAAGCGAAGAACC ATCACCGTGAAGCCTTTGAAG CHKΑ CTTGGTGATGAGCCTCGGAA AAGTGACCTCTCTGCGAGAA CHKB AGTCTCGGTTCCAGTTCTAC CTTCTGCTCGTTGTTCCTCC β ACTIN CCAACCGCGAGAAGATGACC GGAGTCCATCACGATGCCAG HSP90 CCAGTTTGGTGTCGGTTTCTAT CTGGGTATCGTTGTTGTGTTTTG JFH1 Hepatitis C Virus GTCTGCGGAACCGGTGAGTA GCCCAAATGGCCGGGATA Viruses 2020, 12, x; doi: FOR PEER REVIEW www.mdpi.com/journal/viruses Viruses 2020, 12, x FOR PEER REVIEW 2 of 3 Supplementary Figure S1. FBS-cultured Huh7.5 choline transporter transcript expression Supplementary Figure S1. FBS-cultured Huh7.5 choline transporter transcript expression. RNA was isolated from FBS-cultured Huh7.5 cells to measure choline transporter (SLC44A1-5; choline transporter-like 1-5, and SLC22A1 and 2; organic cation transporter 1 and 2) transcript expression. All groups are shown relative to the expression of SLC44A1 and normalized to the average of β ACTIN and HSP90. Supplementary Figure S2. HS-cultured Huh7.5 choline uptake kinetics Supplementary Figure S2. HS-cultured Huh7.5 choline uptake kinetics. Choline uptake saturation kinetics from HS-cultured Huh7.5 cells, fit to a Michaelis-Menten curve. Data are mean±SEM and are representative of 3 independent experiments. Viruses 2020, 12, x FOR PEER REVIEW 3 of 3 Supplementary Figure S3. Inhibition of choline transport in FBS-cultured infected cells Supplementary Figure S3. Inhibition of choline transport in FBS-cultured infected cells. FBS-cultured Huh7.5 cells were infected at an MOI of 1 for 24 h in the presence or absence of 20 or 200 μM hemicholinium-3 (HC-3) to inhibit choline uptake. -

Therapeutic Effects of Jiaotai Pill on Rat Insomnia Via Regulation of GABA Signal Pathway
Tang et al Tropical Journal of Pharmaceutical Research September 2017; 16 (9): 2135-2140 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v16i9.13 Original Research Article Therapeutic effects of Jiaotai pill on rat insomnia via regulation of GABA signal pathway Na-na Tang1,2, Chang-wen Wu1, Ming-qi Chen3, Xue-ai Zeng3, Xiu-feng Wang3, Yu Zhang3 and Jun-shan Huang1,3* 1Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, 350122, 2Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi, 330004, 3The Sleep Research Center of Fujian Provincial Institute of Traditional Chinese Medicine, Fuzhou, Fujian 350003, China. *For correspondence: Email: [email protected] Sent for review: 27 January 2017 Revised accepted: 5 August 2017 Abstract Purpose: To investigate the therapeutic effects of Jiaotai pill (JTP) on rats with insomnia induced by p- chlorophenylalanine (PCPA). Methods: Rats with PCPA-induced insomnia were divided into 5 groups (n = 10), made up of control group, positive treatment group (estazolam 0.1 mg/kg), and 3 JTP treatment groups (0.6, 1.2 and 2.4 g/kg). Another group of 10 rats were treated as normal group. Rats in normal and control groups were treated with normal saline (10 mL/kg). After 14 days of drug treatment, the rats were injected intraperitoneally with sodium pentobarbital (45 mg/kg) and thereafter, latent period and sleeping time were recorded, while contents of γ-aminobutyric acid (GABA) and glutamic acid (Glu) in hypothalamus were determined by high performance liquid chromatography (HPLC). -

Control of Choline Oxidation in Rat Kidney Mitochondria
ÔØ ÅÒÙ×Ö ÔØ Control of Choline Oxidation in Rat Kidney Mitochondria Niaobh O’Donoghue, Trevor Sweeney, Robin Donagh, Kieran Clarke, Richard K. Porter PII: S0005-2728(09)00144-3 DOI: doi:10.1016/j.bbabio.2009.04.014 Reference: BBABIO 46295 To appear in: BBA - Bioenergetics Received date: 11 March 2009 Revised date: 27 April 2009 Accepted date: 29 April 2009 Please cite this article as: Niaobh O’Donoghue, Trevor Sweeney, Robin Donagh, Kieran Clarke, Richard K. Porter, Control of Choline Oxidation in Rat Kidney Mitochondria, BBA - Bioenergetics (2009), doi:10.1016/j.bbabio.2009.04.014 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. ACCEPTED MANUSCRIPT Control of Choline Oxidation in Rat Kidney Mitochondria Niaobh O’Donoghue, Trevor Sweeney, Robin Donagh, Kieran Clarke and Richard K. Porter * School of Biochemistry and Immunology, Trinity College Dublin, Dublin 2 Ireland. *Correspondence to: Richard K. Porter, School of Biochemistry and Immunology, Trinity College Dublin, Dublin 2 Ireland. Tel. +353-1-8961617; Fax +353-1-6772400; Email: [email protected] Key words: choline, betaine, mitochondria, osmolyte, kidney, transporter ACCEPTED MANUSCRIPT Abbreviations: EGTA, ethylenebis(oxethylenenitrilo)tetraacetic acid; FCCP, carbonyl cyanide p-trifluoromethoxyphenylhyrazone; HEPES, 4-(2- hydroxyethyl)-1-peiperazine-ethanesulfonic acid; MOPS, (3-[N}- morphilino)propane sulphonic acid; TPMP, methyltriphenylphosphonium 1 ACCEPTED MANUSCRIPT ABSTRACT Choline is a quaternary amino cationic organic alcohol that is oxidized to betaine in liver and kidney mitochondria.