Control of Choline Oxidation in Rat Kidney Mitochondria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Concise Guide to Pharmacology 2019/20
Edinburgh Research Explorer THE CONCISE GUIDE TO PHARMACOLOGY 2019/20 Citation for published version: Cgtp Collaborators 2019, 'THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters', British Journal of Pharmacology, vol. 176 Suppl 1, pp. S397-S493. https://doi.org/10.1111/bph.14753 Digital Object Identifier (DOI): 10.1111/bph.14753 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: British Journal of Pharmacology General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 28. Sep. 2021 S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology (2019) 176, S397–S493 THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters Stephen PH Alexander1 , Eamonn Kelly2, Alistair Mathie3 ,JohnAPeters4 , Emma L Veale3 , Jane F Armstrong5 , Elena Faccenda5 ,SimonDHarding5 ,AdamJPawson5 , Joanna L -

Compositions and Methods for Selective Delivery of Oligonucleotide Molecules to Specific Neuron Types
(19) TZZ ¥Z_T (11) EP 2 380 595 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 26.10.2011 Bulletin 2011/43 A61K 47/48 (2006.01) C12N 15/11 (2006.01) A61P 25/00 (2006.01) A61K 49/00 (2006.01) (2006.01) (21) Application number: 10382087.4 A61K 51/00 (22) Date of filing: 19.04.2010 (84) Designated Contracting States: • Alvarado Urbina, Gabriel AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Nepean Ontario K2G 4Z1 (CA) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Bortolozzi Biassoni, Analia Alejandra PT RO SE SI SK SM TR E-08036, Barcelona (ES) Designated Extension States: • Artigas Perez, Francesc AL BA ME RS E-08036, Barcelona (ES) • Vila Bover, Miquel (71) Applicant: Nlife Therapeutics S.L. 15006 La Coruna (ES) E-08035, Barcelona (ES) (72) Inventors: (74) Representative: ABG Patentes, S.L. • Montefeltro, Andrés Pablo Avenida de Burgos 16D E-08014, Barcelon (ES) Edificio Euromor 28036 Madrid (ES) (54) Compositions and methods for selective delivery of oligonucleotide molecules to specific neuron types (57) The invention provides a conjugate comprising nucleuc acid toi cell of interests and thus, for the treat- (i) a nucleic acid which is complementary to a target nu- ment of diseases which require a down-regulation of the cleic acid sequence and which expression prevents or protein encoded by the target nucleic acid as well as for reduces expression of the target nucleic acid and (ii) a the delivery of contrast agents to the cells for diagnostic selectivity agent which is capable of binding with high purposes. -

Interplay Between Metformin and Serotonin Transport in the Gastrointestinal Tract: a Novel Mechanism for the Intestinal Absorption and Adverse Effects of Metformin
INTERPLAY BETWEEN METFORMIN AND SEROTONIN TRANSPORT IN THE GASTROINTESTINAL TRACT: A NOVEL MECHANISM FOR THE INTESTINAL ABSORPTION AND ADVERSE EFFECTS OF METFORMIN Tianxiang Han A dissertation submitted to the faculty of the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Eshelman School of Pharmacy. Chapel Hill 2013 Approved By: Dhiren R. Thakker, Ph.D. Michael Jay, Ph.D. Kim L. R. Brouwer, Pharm.D., Ph.D. Joseph W. Polli, Ph.D. Xiao Xiao, Ph.D. © 2013 Tianxiang Han ALL RIGHTS RESERVED ii ABSTRACT TIANXIANG HAN: Interplay between Metformin and Serotonin Transport in the Gastrointestinal Tract: A Novel Mechanism for the Intestinal Absorption and Adverse Effects of Metformin (Under the direction of Dhiren R. Thakker, Ph.D.) Metformin is a widely prescribed drug for Type II diabetes mellitus. Previous studies have shown that this highly hydrophilic and charged compound traverses predominantly paracellularly across the Caco-2 cell monolayer, a well-established model for human intestinal epithelium. However, oral bioavailability of metformin is significantly higher than that of the paracellular probe, mannitol (~60% vs ~16%). Based on these observations, the Thakker laboratory proposed a “sponge” hypothesis (Proctor et al., 2008) which states that the functional synergy between apical (AP) transporters and paracellular transport enhances the intestinal absorption of metformin. This dissertation work aims to identify AP uptake transporters of metformin, determine their polarized localization, and elucidate their roles in the intestinal absorption and adverse effects of metformin. Chemical inhibition and transporter-knockdown studies revealed that four transporters, namely, organic cation transporter 1 (OCT1), plasma membrane monoamine transporter (PMAT), serotonin reuptake transporter (SERT) and choline high-affinity transporter (CHT) contribute to AP uptake of metformin in Caco-2 cells. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Prokaryotic Solute/Sodium Symporters: Versatile Functions and Mechanisms of a Transporter Family †
Review Prokaryotic Solute/Sodium Symporters: Versatile Functions and Mechanisms of a Transporter Family † Tania Henriquez, Larissa Wirtz, Dan Su and Heinrich Jung * Microbiology, Department Biology 1, Ludwig Maximilians University Munich, D-82152 Martinsried, Germany; [email protected] (T.H.); [email protected] (L.W.); [email protected] (D.S.) * Correspondence: [email protected]; Tel.: +49-89-218074630 † This article is dedicated to the memory of Ron Kaback, an exceptional scientist and great mentor. Abstract: The solute/sodium symporter family (SSS family; TC 2.A.21; SLC5) consists of integral membrane proteins that use an existing sodium gradient to drive the uphill transport of various solutes, such as sugars, amino acids, vitamins, or ions across the membrane. This large family has representatives in all three kingdoms of life. The human sodium/iodide symporter (NIS) and the sodium/glucose transporter (SGLT1) are involved in diseases such as iodide transport defect or glu- cose-galactose malabsorption. Moreover, the bacterial sodium/proline symporter PutP and the so- dium/sialic acid symporter SiaT play important roles in bacteria–host interactions. This review fo- cuses on the physiological significance and structural and functional features of prokaryotic mem- bers of the SSS family. Special emphasis will be given to the roles and properties of proteins con- taining an SSS family domain fused to domains typically found in bacterial sensor kinases. Citation: Henriquez, T.; Wirtz, L.; Keywords: secondary transport; solute/sodium symport; SLC5; PutP; signal transduction; bacterial Su, D.; Jung, H. Prokaryotic two-component systems; bacterial sensor kinase Solute/Sodium Symporters: Versatile Functions and Mechanisms of a Transporter Family. -

Molecular Characterization of Neurotransmitter Transporters
Molecular Characterization of Neurotransmitter Transporters Saad Shafqat, Maria Velaz-Faircloth, Ana Guadaiio-Ferraz, and Robert T. Fremeau, Jr. Downloaded from https://academic.oup.com/mend/article/7/12/1517/2714704 by guest on 06 October 2020 Departments of Pharmacology and Neurobiology Duke University Medical Center Durham, North Carolina 27710 INTRODUCTION ogical processes. For example, blockade and/or rever- sal of the high affinity plasma membrane L-glutamate transporter during ischemia or anoxia elevates the ex- Rapid chemical signaling between neurons and target tracellular concentration of L-glutamate to neurotoxic cells is dependent upon the precise control of the levels resulting in nerve cell damage (7). Conversely, concentration and duration of neurotransmitters in syn- specific GABA uptake inhibitors are being developed as aptic spaces. After transmitter release from activated potential anticonvulsant and antianxiety agents (8). The nerve terminals, the principal mechanism involved in the ability of synaptic transporters to accumulate certain rapid clearance from the synapse of the biogenic amine neurotransmitter-like toxins, including N-methyW and amino acid neurotransmitters is active transport of phenylpyridine (MPP+), 6-hydroxydopamine, and 5,6- the transmitter back into presynaptic nerve terminals or dihydroxytryptamine, suggests a role for these active glial surrounding cells by one of a large number of transport proteins in the selective vulnerability of neu- specific, pharmacologically distinguishable membrane rons -

GAT-1, a High-Affinity GABA Plasma Membrane Transporter, Localized to Neurons and Astroglia in the Cerebral Cortex
The Journal of Neuroscience, November 1995, 75(11): 7734-7746 GAT-1, a High-Affinity GABA Plasma Membrane Transporter, Localized to Neurons and Astroglia in the Cerebral Cortex Andrea Minelli,’ Nicholas C. Brecha, 2,3,4,5,6Christine Karschiq7 Silvia DeBiasi,8 and Fiorenzo Conti’ ‘Institute of Human Physiology, University of Ancona, Ancona, Italy, *Department of Neurobiology, 3Department of Medicine, 4Brain Research Institute, and 5CURE:VA/UCLA Gastroenteric Biology Center, UCLA School of Medicine, and 6Veterans Administration Medical Center, Los Angeles, CA, 7Max-Planck-lnstitute for Experimental Medicine, Gottingen, Germany, and 8Department of General Physiology and Biochemistry, Section of Histology and Human Anatomy, University of Milan, Milan, Italy High affinity, GABA plasma membrane transporters influ- into GABAergic axon terminals, GAT-1 influences both ex- ence the action of GABA, the main inhibitory neurotrans- citatory and inhibitory transmission by modulating the mitter. The cellular expression of GAT-1, a prominent “paracrine” spread of GABA (Isaacson et al., 1993), and GABA transporter, has been investigated in the cerebral suggest that astrocytes may play an important role in this cortex of adult rats using in situ hybridizaton with %-la- process. beled RNA probes and immunocytochemistry with affinity [Key words: GABA, GABA transporlers, neocottex, syn- purified polyclonal antibodies directed to the C-terminus of apses, neurons, astrocytes] rat GAT-1. GAT-1 mRNA was observed in numerous neurons and in Synaptic transmissionmediated by GABA plays a key role in some glial cells. Double-labeling experiments were per- controlling neuronal activity and information processingin the formed to compare the pattern of GAT-1 mRNA containing mammaliancerebral cortex (Krnjevic, 1984; Sillito, 1984; Mc- and GAD67 immunoreactive cells. -
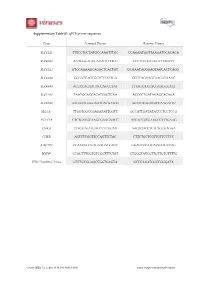
Gene Forward Primer Reverse Primer
Supplementary Table S1. qPCR primer sequences. Gene Forward Primer Reverse Primer SLC44A1 TTTCCTGCTATGCCAAGTTTGC CCAGAATGGTTAAGATCCACACA SLC44A2 AAAGGGAGGGAGAGTTTTGC CCCTTGGGTGGGTTTAGTTT SLC44A3 GTCCAAAAGCAGACTCACTGT GCAAATAGGGAGTAGCACTCAGG SLC44A4 GGGATCAGCGGTCTTATTGA GCGCAGAAGCAAGATAAAC SLC44A5 ACCCCAGAAGAGCAGCCTAT TTTAGCAACACGGAGGGACT SLC22A1 TAATGGACCACATCGCTCAA ACCCCTGATAGAGCACAGA SLC22A2 AAGAATGGGGAATCACAATGG AGATGTGGACGCCAAGATTC SLC5A7 TTGGTGGCCGAGATATTGGTT GCCATTGATATACCCTCCTCCG PCYT1A CTCTGATGCAAGCGAAGAACC ATCACCGTGAAGCCTTTGAAG CHKΑ CTTGGTGATGAGCCTCGGAA AAGTGACCTCTCTGCGAGAA CHKB AGTCTCGGTTCCAGTTCTAC CTTCTGCTCGTTGTTCCTCC β ACTIN CCAACCGCGAGAAGATGACC GGAGTCCATCACGATGCCAG HSP90 CCAGTTTGGTGTCGGTTTCTAT CTGGGTATCGTTGTTGTGTTTTG JFH1 Hepatitis C Virus GTCTGCGGAACCGGTGAGTA GCCCAAATGGCCGGGATA Viruses 2020, 12, x; doi: FOR PEER REVIEW www.mdpi.com/journal/viruses Viruses 2020, 12, x FOR PEER REVIEW 2 of 3 Supplementary Figure S1. FBS-cultured Huh7.5 choline transporter transcript expression Supplementary Figure S1. FBS-cultured Huh7.5 choline transporter transcript expression. RNA was isolated from FBS-cultured Huh7.5 cells to measure choline transporter (SLC44A1-5; choline transporter-like 1-5, and SLC22A1 and 2; organic cation transporter 1 and 2) transcript expression. All groups are shown relative to the expression of SLC44A1 and normalized to the average of β ACTIN and HSP90. Supplementary Figure S2. HS-cultured Huh7.5 choline uptake kinetics Supplementary Figure S2. HS-cultured Huh7.5 choline uptake kinetics. Choline uptake saturation kinetics from HS-cultured Huh7.5 cells, fit to a Michaelis-Menten curve. Data are mean±SEM and are representative of 3 independent experiments. Viruses 2020, 12, x FOR PEER REVIEW 3 of 3 Supplementary Figure S3. Inhibition of choline transport in FBS-cultured infected cells Supplementary Figure S3. Inhibition of choline transport in FBS-cultured infected cells. FBS-cultured Huh7.5 cells were infected at an MOI of 1 for 24 h in the presence or absence of 20 or 200 μM hemicholinium-3 (HC-3) to inhibit choline uptake. -

The Opu Family of Compatible Solute Transporters from Bacillus Subtilis
Biol. Chem. 2017; 398(2): 193–214 Review Tamara Hoffmann and Erhard Bremer* Guardians in a stressful world: the Opu family of compatible solute transporters from Bacillus subtilis DOI 10.1515/hsz-2016-0265 Received August 8, 2016; accepted August 29, 2016; previously Introduction published online December 8, 2016 Bacillus subtilis, the model organism for Gram-positive Abstract: The development of a semi-permeable cyto- bacteria, is ubiquitously distributed in the environment, plasmic membrane was a key event in the evolution of and can be found in terrestrial, in plant-associated, and in microbial proto-cells. As a result, changes in the exter- marine ecoystems (Earl et al., 2008; Mandic-Mulec et al., nal osmolarity will inevitably trigger water fluxes along 2015). One of its main habitats is the upper layer of the the osmotic gradient. The ensuing osmotic stress has soil. The functional annotation of its 4.2 Mbp genome consequences for the magnitude of turgor and will nega- sequence (Barbe et al., 2009) shows that it is well adapted tively impact cell growth and integrity. No microorganism to life in this habitat through an abundance of uptake and can actively pump water across the cytoplasmic mem- catabolic systems allowing it to take advantage of many brane; hence, microorganisms have to actively adjust the plant-derived compounds for growth (Belda et al., 2013). B. osmotic potential of their cytoplasm to scale and direct subtilis can exist in the soil as motile cells, actively seeking water fluxes in order to prevent dehydration or -

Sodium-Coupled Glucose Transport, the SLC5 Family, and Therapeutically Relevant Inhibitors: from Molecular Discovery to Clinical Application
Pflügers Archiv - European Journal of Physiology (2020) 472:1177–1206 https://doi.org/10.1007/s00424-020-02433-x INVITED REVIEW Sodium-coupled glucose transport, the SLC5 family, and therapeutically relevant inhibitors: from molecular discovery to clinical application Gergely Gyimesi1 & Jonai Pujol-Giménez1 & Yoshikatsu Kanai2 & Matthias A. Hediger1 Received: 4 March 2020 /Revised: 24 June 2020 /Accepted: 2 July 2020 / Published online: 7 August 2020 # The Author(s) 2020 Abstract Sodium glucose transporters (SGLTs) belong to the mammalian solute carrier family SLC5. This family includes 12 different members in human that mediate the transport of sugars, vitamins, amino acids, or smaller organic ions such as choline. The SLC5 family belongs to the sodium symporter family (SSS), which encompasses transporters from all kingdoms of life. It furthermore shares similarity to the structural fold of the APC (amino acid-polyamine-organocation) transporter family. Three decades after the first molecular identification of the intestinal Na+-glucose cotransporter SGLT1 by expression cloning, many new discoveries have evolved, from mechanistic analysis to molecular genetics, structural biology, drug discovery, and clinical applications. All of these advances have greatly influenced physiology and medicine. While SGLT1 is essential for fast absorption of glucose and galactose in the intestine, the expression of SGLT2 is largely confined to the early part of the kidney proximal tubules, where it reabsorbs the bulk part of filtered glucose. SGLT2 has been successfully exploited by the pharmaceutical industry to develop effective new drugs for the treatment of diabetic patients. These SGLT2 inhibitors, termed gliflozins, also exhibit favorable nephroprotective effects and likely also cardioprotective effects. -

Choline Transport and Metabolism in Genetically Deficient and Chronic Disease States
Choline Transport and Metabolism in Genetically Deficient and Chronic Disease States by Laila Cigana Schenkel A Thesis presented to The University of Guelph In partial fulfilment of requirements for the degree of Doctor of Philosophy in Human Health and Nutritional Sciences Guelph, Ontario, Canada © Laila Cigana Schenkel, August, 2014 ABSTRACT CHOLINE TRANSPORT AND METABOLISM IN GENETICALLY DEFICIENT AND CHRONIC DISEASE STATES Laila Cigana Schenkel Advisor: University of Guelph, 2014 Marica Bakovic Choline is required for the biosynthesis of phosphatidylcholine (PC) by the CDP-choline Kennedy pathway and of betaine. Choline also plays a role in lipid metabolism and hepatic and muscle function. The availability of intracellular choline is regulated by the choline transporter CTL1/SLC44A1 at the plasma membrane. This thesis aims to elucidate the effect of metabolic altered states, such as lipid overload, choline deficiency and CDP:phopshoethanolamine cytidylyltransferase (Pcyt2) genetic modified models, on the choline transport and metabolism. First, we investigated the effect of high fatty acid supply in C2C12 muscle cells. Palmitic acid (PAM) reduced total and plasma membrane CTL1/SLC44A1 protein by activating lysosomal degradation, and limited the choline uptake while increasing diacylglycerol (DAG) and triacylglycerol (TAG) synthesis. Oleic acid (OLA) maintained total and plasma membrane CTL1/SLC44A1, increasing PC and TAG synthesis more than PAM, which offers a protection mechanism from the excess of intracellular DAG and autophagy. We next characterized the choline metabolic defect in fibroblast cells isolated from a Postural Tachycardia Syndrome (POTS) patient, who had low plasma choline. In the POTS fibroblasts, the CTL1/SLC44A1 expression and choline uptake were decreased. PC synthesis and the phospholipid pool were reduced these cells compared to control. -

Human Breast Cancer Metastases to the Brain Display Gabaergic Properties in the Neural Niche
Human breast cancer metastases to the brain display GABAergic properties in the neural niche Josh Nemana, John Terminib, Sharon Wilczynskic, Nagarajan Vaidehid, Cecilia Choya,e, Claudia M. Kowolikc, Hubert Lid,e, Amanda C. Hambrechta,f, Eugene Robertsg,1, and Rahul Jandiala,f,1 Divisions of aNeurosurgery and cPathology, Departments of bMolecular Medicine, dImmunology, and gNeurobiochemistry, and eIrell and Manella Graduate School of Biological Sciences, City of Hope, Duarte, CA 91010; and fDepartment of Biology, University of Southern California, Los Angeles, CA 90089 Contributed by Eugene Roberts, November 27, 2013 (sent for review October 16, 2013) Dispersion of tumors throughout the body is a neoplastic process secondary sites (10–14). We previously showed that metastatic responsible for the vast majority of deaths from cancer. Despite cells have the ability to alter the cellular milieu of the brain disseminating to distant organs as malignant scouts, most tumor for growth advantage (3). cells fail to remain viable after their arrival. The physiologic mi- γ-Aminobutyric acid (GABA) was first identified in the croenvironment of the brain must become a tumor-favorable mi- mammalian brain over one-half a century ago and subsequent croenvironment for successful metastatic colonization by circulating studies have demonstrated its relevance to various medical and breast cancer cells. Bidirectional interplay of breast cancer cells and scientific paradigms (15–18). In addition to its role in neuro- native brain cells in metastasis is poorly understood and rarely transmission, GABA can act as a trophic factor during nervous studied. We had the rare opportunity to investigate uncommonly system development to influence cellular events including pro- available specimens of matched fresh breast-to-brain metastases liferation, migration, differentiation, synapse maturation, and tissue and derived cells from patients undergoing neurosurgical re- cell death (19, 20).