Chemical and Physical Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MATERIAL SAFETY DATA SHEET Potassium Sulfate
MATERIAL SAFETY DATA SHEET Potassium sulfate Section 1 Chemical Product and Company Identification MSDS Name: Potassium sulfate Catalog Numbers: P/6960/53, P/6960/60, P/6960/65, P/7000/53, P/7000/60, P/7000/65 Synonyms: Dipotassium sulfate; Potassium sulfate (2:1); Sulfuric acid, dipotassium salt. Company Identification: Fisher Scientific UK Bishop Meadow Road, Loughborough Leics. LE11 5RG For information in Europe, call: (01509) 231166 Emergency Number, Europe: 01509 231166 Section 2 Composition, Information on Ingredients CAS# Chemical Name: % EINECS# 7778805 Potassium sulfate >99 2319155 Hazard Symbols: None listed Risk Phrases: None listed Section 3 Hazards Identification EMERGENCY OVERVIEW Not available Potential Health Effects Eye: Dust may cause mechanical irritation. Skin: Low hazard for usual industrial handling. Ingestion: May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Inhalation: Inhalation of dust may cause respiratory tract irritation. Chronic: Not expected to be a chronic hazard. Section 4 First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid. Skin: In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse. Ingestion: If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid. Inhalation: If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Notes to Treat symptomatically and supportively. Physician: Section 5 Fire Fighting Measures General As in any fire, wear a selfcontained breathing apparatus in pressuredemand, Information: MSHA/NIOSH (approved or equivalent), and full protective gear. -

Sodium Chromate 10 Percent Section 1
Conforms to US OSHA Hazard Communication 29CFR1910.1200 SAFETY DATA SHEET Sodium Chromate 10 percent Section 1. Identification 1.1 Product identifier Product name : Sodium Chromate 10 percent Part no. : AR176, AR376 Validation date : 6/24/2020 1.2 Relevant identified uses of the substance or mixture and uses advised against Material uses : Laboratory use Container type: Dispenser Pack AR176 // Sodium Chromate 10 percent // Artisan Grocott's Methenamine Silver Stain Kit // 65 mL and 115 mL AR376 // Sodium Chromate 10 percent // Artisan Grocott's Methenamine Silver Eosin Stain Kit // 65 mL and 115 mL Reference number: SDS056 1.3 Details of the supplier of the safety data sheet Supplier/Manufacturer : Agilent Technologies, Inc. 5301 Stevens Creek Blvd Santa Clara, CA 95051, USA Tel: +1 800 227 9770 Agilent Technologies Singapore (International) Pte Ltd. No. 1 Yishun Avenue 7 Singapore, 768923 Tel. (65) 6276 2622 Agilent Technologies Denmark ApS Produktionsvej 42 2600 Glostrup, Denmark Tel. +45 44 85 95 00 www.Agilent.com e-mail address of person : [email protected] responsible for this SDS 1.4 Emergency telephone number In case of emergency : CHEMTREC®: 1-800-424-9300 Section 2. Hazards identification 2.1 Classification of the substance or mixture OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the substance or mixture Date of issue : 06/24/2020 1/15 Sodium Chromate 10 percent Section 2. Hazards identification H302 ACUTE TOXICITY (oral) - Category 4 H311 -

Managing Potassium for Organic Crop Production by Robert Mikkelsen an Adequate K Supply Is Essential for Both Organic and Conventional Crop Production
NORTH AMERICA Managing Potassium for Organic Crop Production By Robert Mikkelsen An adequate K supply is essential for both organic and conventional crop production. Potas- sium is involved in many plant physiological reactions, including osmoregulation, protein synthesis, enzyme activation, and photosynthate translocation. The K balance on many farms is negative, where more K is removed in harvested crops than is returned again to the soil. An overview of commonly used K fertilizers for organic production is provided. otassium is an essential nutrient for plant growth, but it often receives less attention than N and P in many crop Pproduction systems. Many regions of the U.S.A. and all of the Canadian provinces remove more K during harvest than is returned to the soil in fertilizer and manure (Figure 1). In the U.S.A., an average of only 3 units of K is replaced as fertilizer and manure for every 4 units of K removed in crops, resulting in a depletion of nutrients from the soil and increasing occur- rences of deficiency in many places. Potassium is the soil cation required in the largest amount by plants, regardless of nutrient management philosophy. 1,400 Removal 1,200 Hay and forage crops can remove hundreds of pounds of K from the soil Manure each year, placing a heavy demand on soil resources. 1,000 Fertilizer Large amounts of K are required to maintain plant health 800 and vigor. Some specific roles of K in the plant include os- moregulation, internal cation/anion balance, enzyme activa- 600 tion, proper water relations, photosynthate translocation, and 400 protein synthesis. -

Packet of Wiser Reports on Acetone Acetonitrile
Ac&tone ^Hazmat - NFPA Hazard Classification Page 1 of Acetone CAS RN: 67-64-1 Hazmat - NFPA Hazard Classification SOMS DocID 2085807 Health: 1 (Slight) Materials that, on exposure, would cause significant irritation, but only minor residual injury, including those requiring the use of an approved air-purifying respirator. These materials are only slightly hazardous to health and only breathing protection is needed. Flammability: 3 (Severe) rhis degree includes Class IB and 1C flammable liquids and materials that can be easily ignited under almost all normal temperature conditions. Water may be ineffective in controlling or extinguishing fires in such materials. Instability: 0 (Minimal) This degree includes materials that are normally stable, even under fire exposure conditions, and that do not react with water.- Norma lire fighting procedures may be used. Printed by WISER for Windows (v2.3.231, database v2.108) HHS/NIH, National Library of Medicine AR000018 iile://C:\Documents and Settings\Gham\Application Data\National Library of Medicine\WISER\2.3.231.628... 9/27/20 Acetone ^Key Info Page 1 of Acetone CAS RN: 67-64-1 Key Info FLAMMABLE LIQUIDS (Polar / Water-Miscible) • HIGHLY FLAMMABLE: Easily ignited by heat, sparks or flames • CAUTION: Very low flash point; use of water spray when fighting fire may be inefficient Printed by WISER for Windows (v2.3.231, database v2.108) HHS/NIH, National Library of Medicine AR000019 file://C:\Documents and Settings\Gham\Application Data\National Library of Medicine\WISER\2.3.231.628../ 9/27/20 Acetone - -Hazmat - Explosive Limits / Potential Page 1 of Acetone CAS RIM: 67-64-1 Hazmat - Explosive Limits / Potential Highly flammable liquid. -

Wood Modified by Inorganic Salts: Mechanism and Properties
WOOD MODIFIED BY INORGANIC SALTS: MECHANISM AND PROPERTIES. I. WEATHERING RATE, WATER REPELLENCY, AND IIIMENSIONAL STABILITY OF WOOD MODIFIEID WITH CHROMIUM (111) NITRATE VERSUS CHROMIC ACID' R. S. Willianzs and W. C. Feisl Research Chemists Forest Products Lab~ratory,~Forest Service U.S. Department of Agriculture Madison, WI 53705 (Received October 1983) ABSTRACT Chromic acid treatment of wood improves surface characteristics. A simple dip or brush application of 5% aqueous chromic acid to a wood surface stops extractive staining, improves dimensional stability, retards weathering of unfinished wood, and prolongs the life of finishes. A better understanding of how chromic acid effects these improvements may facilitate the development of even better treatments. The improvements may be due to a combination offactors: oxidation ofwood by hexavalent chromium compounds, formation of coordination coml~lexeswith trivalent chromium, and the presence of insoluble chromium compounds at the surface. Most trivalent chromium compounds do not become fixed in wood (i.e., do not form water-insolublf: wood-chemical complexes). However, chromium (111) nitrate treatment of western redcedar (Thuja plicata), southern pine (Pinus sp.), and ponderosa pine (Pinus ponderosa) produced modified wood with properties similar to chromic acid-treated wood. Evaluation of the chromium (111) nitrate- and chromic acid-modified wood by leaching, Xenon arc- accelerated weathering, and swellometer experiments clearly demonstrated similar fixation, erosion, and water-repellent properties. Based on the results from chromium (111) nitrate-treated wood, fixation through coordination of trivalent chromium with wood hydroxyls an(5 formation of insoluble chromium appear to be the critical factors in improving the performance of the treated wood. -

(VI) and Chromium (V) Oxide Fluorides
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses 1976 The chemistry of chromium (VI) and chromium (V) oxide fluorides Patrick Jay Green Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Chemistry Commons Let us know how access to this document benefits ou.y Recommended Citation Green, Patrick Jay, "The chemistry of chromium (VI) and chromium (V) oxide fluorides" (1976). Dissertations and Theses. Paper 4039. https://doi.org/10.15760/etd.5923 This Thesis is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. All ABSTRACT OF THE TllESIS OF Patrick Jay Green for the Master of Science in Chemistry presented April 16, 1976. Title: Chemistry of Chromium(VI) and Chromium(V) Oxide Fluorides. APPROVEO BY MEMBERS OF THE THESIS CO'"o\l TIEE: y . • Ii . ' I : • • • • • New preparative routes to chromyl fluoride were sought. It was found that chlorine ironofluoride reacts with chromium trioxide and chromyl chlo ride to produce chromyl fluoride. Attempts were ~ade to define a mechan ism for the reaction of ClF and Cr0 in light of by-products observed 3 and previous investigations. Carbonyl fluoride and chromium trioxide react to fom chro·yl fluoride and carbo:i dioxide. A mechanism was also proposed for this react10n. Chromium trioxide 11itl\ l~F6 or WF5 reacts to produce chromyl fluoride and the respective oxide tetrafluoride. 2 Sulfur hexafluoride did not react with Cr03. -

Molybdates: an Alternative to Hexavalent Chromates in Corrosion
Molybdates: An Alternative to Hexavalent Chromates 3ytzt7n2 in Corrosion Prevention and Control qz ,+ B3 F Stiles C. Thompson Sverdrup Technology, Inc. 626 Anchors St. NW Fort Walton Beach, FL 32548 (904) 833-7600 Introduction Hexavalent chromates have been the preferred corrosion inhibitor in military systems for years. Chromate conversion coatings are specified in MIL-STD-808, “Finishes, Materials and Processes for Corrosion Prevention and Control in Support Equipment ”, for aluminum, magnesium, and plated steel. A number of primers are formulated with zinc or strontium chromate. Passivation treatments for stainless steels frequently specify chromic acid. Some anodic coatings specify baths containing chromic and sulfuric acid and a pcst treatment or rinse with aqueous sodium dichromate. Even water-based primers formulated for volatile organic I compound (VOC) compliance in such areas as southern California contain chromate-based corrosion inhibitors. Chromates are decreasing in industrial use, however, as concerns about their environmental risks and toxicity increase. One pollution prevention approach is to substitute analogs of chromates in similar applications. Molybdate is one such analog of lower toxicity. Federal Environmental Regulations A number of environmental regulations now control hexavalent chromate. Toxic Substances Control Act (TSCA). Under TSCA, the Environmental Protection Agency @PA) must be notified 90 days prior to manufacture. EPA may restrict or prohibit manufacture where there is unreasonable risk to the environment. EPA also has the authority to require testing for health or environmental effects. No current regulation specifies molybdate. Resource Conservation and Recovery Act (RCRA). RCRA regulates the treatment, storage, transportation, and disposal of hazardous waste (HW). The EPA HW number for hexavalent chromium is D007. -

Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium
Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium Glyn R. Fox Environmental Management Branch Environmental Protection Division September 24, 2007 Victoria, British Columbia Table of Contents Page 1. Introduction ……………………………………………………………………… 4 2. Details Related to Derivation of Proposed 2007 Draft Matrix Soil Standards 2.1 Derivation of Human Health Protection Standards – Intake of contaminated soil ………………………………………………………….. 7 2.2 Derivation of Human health Protection Standards – Groundwater used for drinking water ……………………………….…………….......... 7 2.3 Derivation of Environmental Protection Standards – Toxicity to soil invertebrates and plants ……………………………………….......... 9 2.4 Derivation of Environmental Protection Standards – Livestock ingesting soil and fodder ………………………………………………… 9 2.5 Derivation of Environmental Protection Standards – Major microbial function impairment ………………………………….……… 10 2.6 Derivation of Environmental Protection Standards – Groundwater flow to surface water used by aquatic life ..…………………………… 10 2.7 Derivation of Environmental Protection Standards – Groundwater used for livestock watering ……………………………………………… 11 2.8 Derivation of Environmental Protection Standards – Groundwater used for irrigation ………………………………………………………… 12 2.9 CSST “Background” Adjustment ……………………..……………… 12 2.10 Application of CSST Rounding-off Rule ……………………………… 12 3. References …….…………………………………………………………………… 13 2 Table of Contents (continued) Page 4. Exhibits Exhibit 1. Proposed 2007 Draft Matrix Soil Standards for Barium ……..… 19 5. Tables -

A Titrimetric Determination of Thorium John J
Ames Laboratory ISC Technical Reports Ames Laboratory 6-1954 A titrimetric determination of thorium John J. Ford Iowa State College James S. Fritz Iowa State College Follow this and additional works at: http://lib.dr.iastate.edu/ameslab_iscreports Part of the Chemistry Commons Recommended Citation Ford, John J. and Fritz, James S., "A titrimetric determination of thorium" (1954). Ames Laboratory ISC Technical Reports. 92. http://lib.dr.iastate.edu/ameslab_iscreports/92 This Report is brought to you for free and open access by the Ames Laboratory at Iowa State University Digital Repository. It has been accepted for inclusion in Ames Laboratory ISC Technical Reports by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. A titrimetric determination of thorium Abstract A rapid, accurate method for thorium is proposed in which thorium is titrated with a standard solution of ethylenediaminetetraacetic acid (EDTA). Alizarin Red S serves as the indicator, a sharp change from pink to yellow marking the end point of the titration. The method is selective for thorium although several cations and anions interfere. A preliminary extraction of thorium nitrate by mentyl oxide provides an excellent separation of thorium from most interfering ions. Keywords Ames Laboratory Disciplines Chemistry This report is available at Iowa State University Digital Repository: http://lib.dr.iastate.edu/ameslab_iscreports/92 Physical 'cieuccs Re· dmg Room UNITED STATES ATOMIC ENERGY COMMISSION ISC-520 A TITRIMETRIC DETERMINATION OF THORIUM By John J. Ford James S. Fritz June 1954 Technical Information Service, Oak Ridge, Tennessee J Subject Category, CHEMISTRY. Work performed under Contract No. -

Chemical Resistance Chart
CHEMICAL RESISTANCE CHART CHEMICAL RESISTANCE DATA These recommendations are based upon information from material suppliers and careful examination of available published information and are believed to be accurate. However, since the resistance of metals, plastics and elastomers can be affected by concentration, temperature, presence of other chemicals and other factors. This information should be considered as a general guide rather than an unqualified guarantee. Ultimately, the customer must determine the suitability of the pump used in various solutions. All recommendations assume ambient temperatures unless otherwise noted. RATINGS — CHEMICAL EFFECT FOOTNOTES A — No effect—Excellent 1. P.V.C. — Satisfactory to 72 °F B — Minor effect—Good 2. Polypropylene — Satisfactory to 72 °F C — Moderate effect—Fair 3. Polypropylene — Satisfactory to 120 °F D — Severe effect—Not recommended 4. Nitrile — Satisfactory for “O” Rings 5. Polyacetal — Satisfactory to 72 °F 6. Ceramag — Satisfactory to 72 °F The ratings for these materials are based upon the chemical resistance only. Added consideration must be given to pump selections when the chemical is abrasive, viscous in nature, or has a Specific Gravity greater than 1.1. NOTE: The materials shown below in BOLDFACE TYPE are used in the construction of Little Giant chemical pumps. ” 302 Stainless Steel 304 Stainless Steel 316 Stainless Steel 440 Stainless Steel Aluminum TITANIUM C276 NICKEL ALLOY Cast Bronze Brass Cast Iron Carbon Steel 1) PVC (Type (E-3606) Tygon PTFE Polyacetal Nylon ABS Thermoplastic -
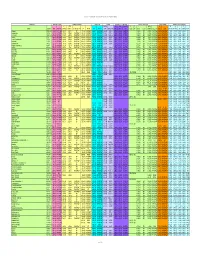
Chemical-Specific Parameters Supporting Table May 2016 Analyte
Regional Screening Level (RSL) Chemical-specific Parameters Supporting Table May 2016 Contaminant Molecular Weight Volatility Parameters Melting Point Density Diffusivity in Air and Water Partition Coefficients Water Solubility Tapwater Dermal Parameters H` (atm- Density Dia Diw Dia and Diw Kd Kd Koc log Kow S B τevent t* Kp 3 3 2 2 Analyte CAS No. MW MW Ref (unitless) m /mole) H` and HLC Ref VP VP Ref MP MP Ref (g/cm ) Density Ref (cm /s) (cm /s) Ref (L/kg) Ref (L/kg) Koc Ref (unitless) log Kow Ref (mg/L) S Ref (unitless) (hr/event) (hr) (cm/hr) K Ref Acephate 30560-19-1 1.8E+02 PHYSPRO 2.0E-11 5.0E-13 EPI 1.7E-06 PHYSPROP 8.8E+01 PHYSPROP 1.4E+00 CRC89 3.7E-02 8.0E-06 WATER9 1.0E+01 EPI -8.5E-01 PHYSPRO 8.2E+05 PHYSPROP 2.1E-04 1.1E+00 2.7E+00 4.0E-05 EPI Acetaldehyde 75-07-0 4.4E+01 PHYSPRO 2.7E-03 6.7E-05 PHYSPROP 9.0E+02 PHYSPROP -1.2E+02 PHYSPROP 7.8E-01 CRC89 1.3E-01 1.4E-05 WATER9 1.0E+00 EPI -3.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.3E-03 1.9E-01 4.5E-01 5.3E-04 EPI Acetochlor 34256-82-1 2.7E+02 PHYSPRO 9.1E-07 2.2E-08 PHYSPROP 2.8E-05 PHYSPROP 1.1E+01 PubChem 1.1E+00 PubChem 2.2E-02 5.6E-06 WATER9 3.0E+02 EPI 3.0E+00 PHYSPRO 2.2E+02 PHYSPROP 3.1E-02 3.4E+00 8.2E+00 5.0E-03 EPI Acetone 67-64-1 5.8E+01 PHYSPRO 1.4E-03 3.5E-05 PHYSPROP 2.3E+02 PHYSPROP -9.5E+01 PHYSPROP 7.8E-01 CRC89 1.1E-01 1.2E-05 WATER9 2.4E+00 EPI -2.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.5E-03 2.2E-01 5.3E-01 5.1E-04 EPI Acetone Cyanohydrin 75-86-5 8.5E+01 PHYSPRO 8.1E-08 2.0E-09 PHYSPROP 3.4E-01 PHYSPROP -1.9E+01 PHYSPROP 9.3E-01 CRC89 8.6E-02 1.0E-05 WATER9 1.0E+00 -

Customer Information Regarding Material Resistance in Compressed Air Preparation
Customer information regarding material resistance in compressed air preparation Polycarbonate reservoirs for filter regulators, filters, and lubricators Everywhere the presence of these media cannot be avoided, such as in paint booths, gluing machines, Polycarbonate is the longest known and most vulcanization plants, etc., the use of metal reservoirs processed material in the world for reservoirs of is required. compressed air maintenance units. The high Problematic is the use of solvents not only in pressure and temperature resistances, as well as immediate contact with the reservoir, but also in its good compatibility with the normally used media immediate vicinity. For example, trichlorethylene such as water, oils and greases are some of its vapors from the compressor’s intake air can cause advantages. crack formation in the polycarbonate reservoir. Only The only “weakness” of this plastic is its clean the reservoirs using a slightly damp cloth. Only susceptibility to media that can be referred to use water to do this and, if necessary, a mild collectively using the term “solvents”. From this detergent without chemical additives. range, the materials relevant to the use of If a lubricator is used, please only use suitable compressed air preparation units are summarized pneumatic oils, e.g. AVENTICS pneumatic oil, order here: no. 8982000010 – 1L. Detergents : Trichloroethylene, (usually from outside) perchloroethylene, In most cases, the use of (pneumatic) oils with benzene, additives, for example antifreeze, results in damage super/regular gasoline to or destruction of the reservoirs and must thus be avoided. Alternatively, we recommend the use of Solvents : Acetone, metal reservoirs. (from outside/inside) paint thinners, alcohols, esters Polycarbonate can react to permanent UV-light irradiation and weathering.