Mussels and Oysters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

{Pdf Epub} Clams, Oysters, & Scallops
Read Ebook {PDF EPUB} CLAMS, OYSTERS, & SCALLOPS (Dominie Marine Life Young Readers) by Dominie Elementary Oysters Medium Fresh Jar - 8 Oz Seafood Service Counter Crab Snow Cluster Alaska Cooked Previously Frozen 1 Count - 0.75 LB Seafood Service Counter Scallop Sea U/10 Fresh - 0.75 LB Shellfish: Oysters and Scallops and Clams . Clams are bivalved mollusks that can live up to 35 years and grow up to five inches in diameter; however, most N.C. clams are harvested when they are two years old at the minimum harvest size of one inch thick. The status of the clam fishery in N.C. is "unknown". Landings have remained fairly steady over the years, but it is felt increased fishing ... Oyster, Clams & Scallops Oyster, Clams & Scallops (68) Filters Clear All. Filters Department. Meat & Seafood (n) Fish & Shellfish (n) Oyster, Clams & Scallops (n) Clear All DONE . Brand Clear. ... Open Nature Sea Scallops North Atlantic Wild Caught Colossal Under 15 Count - 16 Oz Scallops Sea Pieces Frozen - 1 Lb Blue Moon LightSky Beer Craft Wheat 4% ABV In Can - 12-12 Fl. Oz. Budweiser Zero 12pk Can - 12-12 Fl. Oz. May 30, 2018 · SCALLOPS are not all created equal. Of course not many things are, but when it comes to scallops, it’s the size that makes all the difference in the world (or in the sea). There are two main types of scallops: sea and bay. Sea scallops, which are caught in the sea, duh, can be nearly three times the size of bay scallops. Nov 20, 2019 · Scallops are another subclass of clam. -
300 Ways to Cook and Serve Shell Fish
l«r'S'i.''n',.i, M 1 i , , ^' >! '' ' Til I !,' ilj'/i! i ill H'l \ tk\^ 1.1! ii r,*. '» 1" ia;lii;iinP|Mr-^ . ., - .!!Lii<i!l!i r''Rf;illH I ill Class ^T53 Book. ^llT Gopyiight^I^ COPYRIGHT DEPOSrr. 300 WAYS Cook and Sef^ve Shell pish Tei'rapiii Green Turtle Snapper Oysters Oyster Crabs Lobsters Clams Crabs and Shrimps BY H. FRANKLYN HALL Chef Boothby Hotel Company Philadelphia, Pa. PHILADELPHIA, PA., Christian Banner Phint, 1842 Lombard St. f^ TH£ LIBRARY OF CONGRESS, One Copy Received ""AY. 13 1901 Copyright entry CUASS^XXc. No COPY^ Copyright, 1901, by H. Frankl> n Hall, Philadelphia, Pa, HutvoDuction To the possessor or reader of this work, I as- sure you that you will find nothing but plain and simple truths. Any one of the three hundred re- ceipts herein would cost you more than the price of the entire book if separately obtained. Many ladies and gentlemen can testify who have ap- plied to me for the same at Boothby's during the past ten years. I have purposely made them plain and simple, so that not only the lady of the house can understand them, but lo save her an- noyance, the butler, housekeeper or cook, not only the proprietor, steward or chef, but the side cook, all of whom hope to become chefs some day as well. I have purposely used the most simple terms in naming the different ingreiiients and in constructing the receipts, so that they would not confuse and annoy, but prove a help and benefit to all who attempt lo use the same. -
Field Identification Guide to the Living Marine Resources In
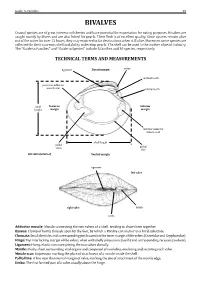
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Attachment Properties of Blue Mussel (Mytilus Edulis L.) Byssus Threads on Culture-Based Artificial Collector Substrates
Aquacultural Engineering 42 (2010) 128–139 View metadata, citation and similar papers at core.ac.uk brought to you by CORE Contents lists available at ScienceDirect provided by Electronic Publication Information Center Aquacultural Engineering journal homepage: www.elsevier.com/locate/aqua-online Attachment properties of blue mussel (Mytilus edulis L.) byssus threads on culture-based artificial collector substrates M. Brenner a,b,c,∗, B.H. Buck a,c,d a Alfred Wegener Institute for Polar and Marine Research (AWI), Am Handelshafen 12, 27570 Bremerhaven, Germany b Jacobs University Bremen, Campus Ring 1, 28759 Bremen, Germany c Institute for Marine Resourses (IMARE), Klußmannstraße 1, 27570 Bremerhaven, Germany d University of Applied Sciences Bremerhaven, An der Karlstadt 8, 27568 Bremerhaven, Germany article info abstract Article history: The attachment strength of blue mussels (Mytilus edulis) growing under exposed conditions on 10 differ- Received 25 May 2009 ent artificial substrates was measured while assessing microstructure of the applied substrate materials. Accepted 9 February 2010 Fleece-like microstructure attracted especially mussel larvae, however, most settled individuals lost attachment on this type of microstructure with increasing size during the time of experiment. Sub- Keywords: strates with thick filaments and long and fixed appendices were less attractive to larvae but provided a Spat collectors better foothold for juvenile mussels as shown by the results of the dislodgement trials. In addition these Offshore aquaculture appendices of substrates could interweave with the mussels, building up a resistant mussel/substrate con- Offshore wind farms Mytilus edulis glomerate. Our results show that a mussel byssus apparatus can withstand harsh conditions, if suitable Dislodgement substrates are deployed. -

Biogeographical Homogeneity in the Eastern Mediterranean Sea. II
Vol. 19: 75–84, 2013 AQUATIC BIOLOGY Published online September 4 doi: 10.3354/ab00521 Aquat Biol Biogeographical homogeneity in the eastern Mediterranean Sea. II. Temporal variation in Lebanese bivalve biota Fabio Crocetta1,*, Ghazi Bitar2, Helmut Zibrowius3, Marco Oliverio4 1Stazione Zoologica Anton Dohrn, Villa Comunale, 80121, Napoli, Italy 2Department of Natural Sciences, Faculty of Sciences, Lebanese University, Hadath, Lebanon 3Le Corbusier 644, 280 Boulevard Michelet, 13008 Marseille, France 4Dipartimento di Biologia e Biotecnologie ‘Charles Darwin’, University of Rome ‘La Sapienza’, Viale dell’Università 32, 00185 Roma, Italy ABSTRACT: Lebanon (eastern Mediterranean Sea) is an area of particular biogeographic signifi- cance for studying the structure of eastern Mediterranean marine biodiversity and its recent changes. Based on literature records and original samples, we review here the knowledge of the Lebanese marine bivalve biota, tracing its changes during the last 170 yr. The updated checklist of bivalves of Lebanon yielded a total of 114 species (96 native and 18 alien taxa), accounting for ca. 26.5% of the known Mediterranean Bivalvia and thus representing a particularly poor fauna. Analysis of the 21 taxa historically described on Lebanese material only yielded 2 available names. Records of 24 species are new for the Lebanese fauna, and Lioberus ligneus is also a new record for the Mediterranean Sea. Comparisons between molluscan records by past (before 1950) and modern (after 1950) authors revealed temporal variations and qualitative modifications of the Lebanese bivalve fauna, mostly affected by the introduction of Erythraean species. The rate of recording of new alien species (evaluated in decades) revealed later first local arrivals (after 1900) than those observed for other eastern Mediterranean shores, while the peak in records in conjunc- tion with our samplings (1991 to 2010) emphasizes the need for increased field work to monitor their arrival and establishment. -

The Peculiar Protein Ultrastructure of Fan Shell and Pearl Oyster Byssus
Soft Matter View Article Online PAPER View Journal | View Issue A new twist on sea silk: the peculiar protein ultrastructure of fan shell and pearl oyster byssus† Cite this: Soft Matter, 2018, 14,5654 a a b b Delphine Pasche, * Nils Horbelt, Fre´de´ric Marin, Se´bastien Motreuil, a c d Elena Macı´as-Sa´nchez, Giuseppe Falini, Dong Soo Hwang, Peter Fratzl *a and Matthew James Harrington *ae Numerous mussel species produce byssal threads – tough proteinaceous fibers, which anchor mussels in aquatic habitats. Byssal threads from Mytilus species, which are comprised of modified collagen proteins – have become a veritable archetype for bio-inspired polymers due to their self-healing properties. However, threads from different species are comparatively much less understood. In particular, the byssus of Pinna nobilis comprises thousands of fine fibers utilized by humans for millennia to fashion lightweight golden fabrics known as sea silk. P. nobilis is very different from Mytilus from an ecological, morphological and evolutionary point of view and it stands to reason that the structure– Creative Commons Attribution 3.0 Unported Licence. function relationships of its byssus are distinct. Here, we performed compositional analysis, X-ray diffraction (XRD) and transmission electron microscopy (TEM) to investigate byssal threads of P. nobilis, as well as a closely related bivalve species (Atrina pectinata) and a distantly related one (Pinctada fucata). Received 20th April 2018, This comparative investigation revealed that all three threads share a similar molecular superstructure Accepted 18th June 2018 comprised of globular proteins organized helically into nanofibrils, which is completely distinct from DOI: 10.1039/c8sm00821c the Mytilus thread ultrastructure, and more akin to the supramolecular organization of bacterial pili and F-actin. -

FAU Institutional Repository
FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©1999 Academic Press. This manuscript is an author version with the final publication available and may be cited as: Young, C. M. (1999). Marine invertebrate larvae. In E. Knobil & J. D. Neill (eds.), Encyclopedia of Reproduction, 3. (pp. 89-97). London, England, and San Diego, CA: Academic Press. --------1111------- Marine Invertebrate Larvae Craig M. Young Harbor Branch Oceanographic Institution 1. What Is a Larva? metamorphOSiS Morphological and physiological changes II. The Production of Larvae that occur during the transition from the larval phase to iII. Larval forms and Diversity the juvenile phase: often coincides with settlement in ben IV. Larval Feeding and Nutrition thic species. V. Larval Orientation, Locomotion, Dispersal, and mixed development A developmental mode that includes a Mortality brooded or encapsulated embryonic stage as well as a free VI. Larval Settlement and Metamorphosis swimming larval stage. VlI. Ecological and Evolutionary Significance of Larvae planktotrophic larva A feeding larva that obtains at least part VlIl. Economic and Medical Importance of Larvae of its nutritional needs from either particulate or dissolved exogenous sources. Planktotrophic larvae generally hatch from small, transparent eggs. GLOSSARY settlement The permanent transition of a larva from the plankton to the benthos. In sessile organisms, settlement atrochal larva A uniformly ciliated larva (cilia not arranged is marked by adhesion to the substratum. It is often closely in distinct bands). associated with metamorphosis and may involve habitat se competent larva A larva that is physiologically and morpho lection. -

Early Ontogeny of Jurassic Bakevelliids and Their Bearing on Bivalve Evolution
Early ontogeny of Jurassic bakevelliids and their bearing on bivalve evolution NIKOLAUS MALCHUS Malchus, N. 2004. Early ontogeny of Jurassic bakevelliids and their bearing on bivalve evolution. Acta Palaeontologica Polonica 49 (1): 85–110. Larval and earliest postlarval shells of Jurassic Bakevelliidae are described for the first time and some complementary data are given concerning larval shells of oysters and pinnids. Two new larval shell characters, a posterodorsal outlet and shell septum are described. The outlet is homologous to the posterodorsal notch of oysters and posterodorsal ridge of arcoids. It probably reflects the presence of the soft anatomical character post−anal tuft, which, among Pteriomorphia, was only known from oysters. A shell septum was so far only known from Cassianellidae, Lithiotidae, and the bakevelliid Kobayashites. A review of early ontogenetic shell characters strongly suggests a basal dichotomy within the Pterio− morphia separating taxa with opisthogyrate larval shells, such as most (or all?) Praecardioida, Pinnoida, Pterioida (Bakevelliidae, Cassianellidae, all living Pterioidea), and Ostreoida from all other groups. The Pinnidae appear to be closely related to the Pterioida, and the Bakevelliidae belong to the stem line of the Cassianellidae, Lithiotidae, Pterioidea, and Ostreoidea. The latter two superfamilies comprise a well constrained clade. These interpretations are con− sistent with recent phylogenetic hypotheses based on palaeontological and genetic (18S and 28S mtDNA) data. A more detailed phylogeny is hampered by the fact that many larval shell characters are rather ancient plesiomorphies. Key words: Bivalvia, Pteriomorphia, Bakevelliidae, larval shell, ontogeny, phylogeny. Nikolaus Malchus [[email protected]], Departamento de Geologia/Unitat Paleontologia, Universitat Autòno− ma Barcelona, 08193 Bellaterra (Cerdanyola del Vallès), Spain. -

Oyster Pdf, Epub, Ebook
OYSTER PDF, EPUB, EBOOK Rebecca Stott | 240 pages | 04 Nov 2004 | Reaktion Books | 9781861892218 | English | London, United Kingdom Oyster PDF Book The Rules of 'Small Ball' This term is batting a thousand. Clam chowder Prep Time. Inspiration and Ideas. By Christine. Flights Vacation Rentals Restaurants Things to do. Discard this shell. City Center West. Rock oysters are available all year round. In general, most types of oyster are eaten raw and this is the most popular way of preparing them. Save Word. The positive ecological and economic results of this are staggering. Oyster sauce is a rich, syruplike sauce that is used in Chinese cuisine. We're intent on clearing it up 'Nip it in the butt' or 'Nip it in the bud'? We had people standing in line. Delicious Oyster Stew This creamy, delicious, and rich oyster stew may not be for traditionalists. Sizes range from 8- ounce, ounce, pint, or gallon containers. The awkward case of 'his or her'. Kiwano Nutrition Facts and Health Benefits. Portuguese 5. There is less fat in oysters than carbohydrates, with just 2 grams per 3 ounces. Reviewed October 26, via mobile Great little place to have dinner. Poor Oyster Casserole Rating: Unrated. Sign up for our free meal planner, click here for more details. The Spruce Eats uses only high-quality sources, including peer-reviewed studies, to support the facts within our articles. Is this restaurant good for bar scene? This is a slight variation on the classic dish Oysters Rockefeller. By Stephen Hart. Dictionary Entries near oyster oy gevalt! We tried the oyster sampler - 3 types of grilled and smoked oysters - out of this world. -

Extensible Byssus of Pinctada Fucata
www.nature.com/scientificreports OPEN Extensible byssus of Pinctada fucata: Ca2+-stabilized nanocavities and a thrombospondin-1 protein Received: 18 June 2015 1,2 1 1 1 1 1 Accepted: 15 September 2015 Chuang Liu , Shiguo Li , Jingliang Huang , Yangjia Liu , Ganchu Jia , Liping Xie & 1 Published: 08 October 2015 Rongqing Zhang The extensible byssus is produced by the foot of bivalve animals, including the pearl oyster Pinctada fucata, and enables them to attach to hard underwater surfaces. However, the mechanism of their extensibility is not well understood. To understand this mechanism, we analyzed the ultrastructure, composition and mechanical properties of the P. fucata byssus using electron microscopy, elemental analysis, proteomics and mechanical testing. In contrast to the microstructures of Mytilus sp. byssus, the P. fucata byssus has an exterior cuticle without granules and an inner core with nanocavities. The removal of Ca2+ by ethylenediaminetetraacetic acid (EDTA) treatment expands the nanocavities and reduces the extensibility of the byssus, which is accompanied by a decrease in the β-sheet conformation of byssal proteins. Through proteomic methods, several proteins with antioxidant and anti-corrosive properties were identified as the main components of the distal byssus regions. Specifically, a protein containing thrombospondin-1 (TSP-1), which is highly expressed in the foot, is hypothesized to be responsible for byssus extensibility. Together, our findings demonstrate the importance of inorganic ions and multiple proteins for bivalve byssus extension, which could guide the future design of biomaterials for use in seawater. In recent years, marine animals have attracted considerable attention in various areas of bioinspired engineering1–4. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

The Effect of Various Spat Collector Materials to Spat Attachment
Journal of Coastal Development ISSN : 1410-5217 Volume 15, Number 1, October 2011 : 34 - 44 Accredited : 83/Dikti/Kep/2009 Original Paper THE EFFECT OF VARIOUS SPAT COLLECTOR MATERIALS FOR SPAT ATTACHMENT OF PEARL OYSTER (Pinctada maxima) Endang Arini1 and Nur Taufiq Syamsudin Putra Jaya2 1Fisheries Department, Faculty of Fisheries and Marine Science, Diponegoro University 2Marine Science Department Faculty of Fisheries and Marine Science, Diponegoro University Jl. Prof. Soedarto, SH, Tembalang Campus, Semarang -50275, Indonesia Received : April, 20, 2011 ; Accepted : June, 30, 2011 ABSTRACT Early stage development of pearl oyster spat was very critical, hence spat required suitable substrates for their settlement to complete their metamorphosis. In this stage, byssus for the attachment is vulnerable and easy to break if there are some disturbance e.g. water movement, hence spat easily falling onto the bottom layer which may have various dirt materials leading to the dead of the spat. In the pearl oyster culture spat collector is important. The aim of this study is to study the effect of different collector materials to the number of spat settled, to reveal the best collector materials and the endurance of spat at the collector. This study was conducted in PT Autore Pearl Culture, at Sumbawa island, Nusa Tenggara Province. Materials used in this research were collectors of different substance i.e. asbestos (A), bamboo (B), polyethylene and roof ceramic (D) substance. The method used was laboratories experiment with Completely Random Design by 4 treatments and 3 replications. Data taken were the number of spat settlement, endurance test, and water quality. Data tested with Analysis of Variance (ANOVA) and further tested with Duncan Duplicate Regional test.