Tracing Shina and Wakhi Origins: Are Ethnic Classifications Commonly Used in Demographic Studies Biologically Meaningful?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cro Ssro Ads Asia
11 How migrants made their way: The role of pioneering migrants and solidarity networks of the Wakhi of Gojal (Northern Pakistan) in shaping the dynamics of rural-urban migration Andreas Benz Working Paper Series Paper Working crossroads asia crossroads ISSN 2192-6034 Bonn, July 2013 Crossroads Asia Working Papers Competence Network Crossroads Asia: Conflict – Migration – Development Editors: Ingeborg Baldauf, Stephan Conermann, Anna-Katharina Hornidge, Hermann Kreutzmann, Shahnaz Nadjmabadi, Dietrich Reetz, Conrad Schetter and Martin Sökefeld. How to cite this paper: Benz, Andreas (2013): How migrants made their way: The role of pioneering migrants and solidarity networks of the Wakhi of Gojal (Northern Pakistan) in shaping the dynamics of rural-urban migration. In: Crossroads Asia Working Paper Series, No. 11. Partners of the Network: Imprint Competence Network Crossroads Asia: Conflict – Migration – Development Project Office Center for Development Research/ZEFa Department of Political and Cultural Change University of Bonn Walter-Flex Str. 3 D-53113 Bonn Tel: + 49-228-731722 Fax: + 49-228-731972 Email: [email protected] Homepage: www.crossroads-asia.de i How migrants made their way: The role of pioneering migrants and solidarity networks of the Wakhi of Gojal (Northern Pakistan) in shaping the dynamics of rural-urban migration Andreas Benz Table of Contents 1. Introduction ..................................................................................................................................... 1 2. The current state -

Grammatical Gender in Hindukush Languages
Grammatical gender in Hindukush languages An areal-typological study Julia Lautin Department of Linguistics Independent Project for the Degree of Bachelor 15 HEC General linguistics Bachelor's programme in Linguistics Spring term 2016 Supervisor: Henrik Liljegren Examinator: Bernhard Wälchli Expert reviewer: Emil Perder Project affiliation: “Language contact and relatedness in the Hindukush Region,” a research project supported by the Swedish Research Council (421-2014-631) Grammatical gender in Hindukush languages An areal-typological study Julia Lautin Abstract In the mountainous area of the Greater Hindukush in northern Pakistan, north-western Afghanistan and Kashmir, some fifty languages from six different genera are spoken. The languages are at the same time innovative and archaic, and are of great interest for areal-typological research. This study investigates grammatical gender in a 12-language sample in the area from an areal-typological perspective. The results show some intriguing features, including unexpected loss of gender, languages that have developed a gender system based on the semantic category of animacy, and languages where this animacy distinction is present parallel to the inherited gender system based on a masculine/feminine distinction found in many Indo-Aryan languages. Keywords Grammatical gender, areal-typology, Hindukush, animacy, nominal categories Grammatiskt genus i Hindukush-språk En areal-typologisk studie Julia Lautin Sammanfattning I den här studien undersöks grammatiskt genus i ett antal språk som talas i ett bergsområde beläget i norra Pakistan, nordvästra Afghanistan och Kashmir. I området, här kallat Greater Hindukush, talas omkring 50 olika språk från sex olika språkfamiljer. Det stora antalet språk tillsammans med den otillgängliga terrängen har gjort att språken är arkaiska i vissa hänseenden och innovativa i andra, vilket gör det till ett intressant område för arealtypologisk forskning. -
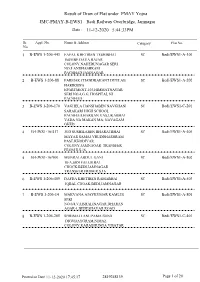
Jmc-Pmay-B-Ews1 11-12-2020 5:44:33Pm
Result of Draw of Flat under PMAY Yojna JMC-PMAY-B-EWS1 Bedi Railway Overbridge, Jamnagar Date : 11-12-2020 5:44:33PM Sr. Appl. No. Name & Address Category Flat No. No. 1 B-EWS 1-206-443 FAFAL KHETIBEN TESHIBHAI SC Bedi/EWS1-A-106 BOMBE DAVA BAJAR COLONY,NAHERUNAGAR SERI NO.5 ANDHASHRAM PACHHAD,JAMNAGAR 2 B-EWS 1-206-88 PARMAR CHANDRAKANT DHULAB SC Bedi/EWS1-A-202 HARIKRIPA EPARTMENT-103,HIMMATNAGAR SERI NO.4,G.G. HOSPITAL NI PACHHAD 3 B-EWS 1-206-170 VAGHELA HANSHABEN NAVGHANB SC Bedi/EWS1-C-201 SARAKARI HIGH SCHOOL PACHHAD,HARIJAN VAS,UKABHAI VADA NA MAKAN MA, NAVAGAM GHED 4 B-EWS1-16/417 JOD SUSHILABEN BHARATBHAI SC Bedi/EWS1-A-406 MAYAR SAMAJ,VRUDDHASHRAM PASE,KHODIYAR COLONY,JAMNAGAR TRANSFER FROM R.S.16 5 B-EWS1-16/900 MUNRAI ABDUL GANI SC Bedi/EWS1-A-802 18-A,SIDI FALI,IKBAL CHOCK,BEDI,JAMNAGAR TRANSFER FROM R.S.16 6 B-EWS 1-206-409 DAFDA KHETIBEN RAMABHAI SC Bedi/EWS1-A-503 IQBAL CHOAK,BEDI,JAMNAGAR 7 B-EWS 1-206-14 MAKVANA AJAYKUMAR KAMLES SC Bedi/EWS1-A-801 SERI NO.6/4,VAISHALINAGAR,DHARAN AGAR-1,BEDESHAVAR ROAD 8 B-EWS 1-206-205 SHRIMALI ANUPAMA SUNIL SC Bedi/EWS1-C-401 DIGVIJAYGRAM,NIMAZ COLONY,KARABHUNGA VISATAR Printed on Date 11-12-2020 17:45:17 2819248139 Page 1 of 20 Result of Draw of Flat under PMAY Yojna JMC-PMAY-B-EWS1 Bedi Railway Overbridge, Jamnagar Date : 11-12-2020 5:44:33PM Sr. -

Gilgit- Baltistan)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Florence Research Naveiñ Reet: Nordic Journal of Law and Social Research (NNJLSR) No.8 2018, pp. 77-100 Civil Society and Governance in Gojal (Gilgit- Baltistan) Fazal Amin Beg 1 and Zoran Lapov 2 Abstract Focusing on both soft and hard development, Aga Khan Rural Support Programme (AKRSP) reached Gilgit-Baltistan and neighbouring Chitral (northern Pakistan) in 1982. In a short span of time, more than 4,500 community associations, namely village and women’s organisations (VWOs), were formed throughout the Region, introducing the local population to democratic governance grounded in civil society-based system. The phenomenon brought about producing significant public and agricultural infrastructures in less than 15 years. As a result, AKRSP turned into a laboratory and model of rural development for many South Asian realities. Underpinned by a qualitative case study and relevant literature review, the present research addresses the process of civil society construction in Gojal (sub-district of Hunza, Gilgit-Baltistan), including the role and engagement of AKRSP: introduced by depicting the scheme of traditional social institutions in front of novel patterns of social organisation, the paper delves into the emergence of – formal and informal – civil society organisations (CSOs) in the form of local support organisations (LSOs) as federations of VWOs and related networks, whose establishment in Gilgit-Baltistan (as well as in Chitral) is a recent phenomenon dating back to the mid-2000s. 1 Fazal Amin Beg, the main author of the paper, has academic background in Sociocultural anthropology, and Farsi literature. -

Open Access Journal Home Page: ISSN: 2223-957X
Int. J. Econ. Environ. Geol. Vol. Alam10 (4 et) 25al.- 29 /Int.J.Econ.Environ.Geol.Vol., 2019 10(4) 25-29, 2019 Open Access Journal home page: www.econ-environ-geol.org ISSN: 2223-957X c The Process Designing of Gold Extraction from Placers of Passu to Shimshal (Hunza Valley) Gilgit-Baltistan by Mercury Amalgamation and Cyanidation Leached Method Muhammad Alam1, Javed Akhter Qureshi2*, Garee Khan2, Manzoor Ali3, Naeem Abbas4, Sher Sultan2, Asghar Khan,2 Yasmeen Bano5 Government Degree College Gilgit,1 Department of Earth Sciences,2 Department of Physics,3 Department of Mining Engineering,4 Department of Mathematical Sciences,5 Karakoram International University, Gilgit, Pakistan *E mail: [email protected] Received: 22 October, 2019 Accepted: 03 January, 2020 Abstract: Gold wash through panning or washing in the fluvial sediments or sands is done on the river banks in Skardu, Hunza and other places. The method of gold washing is very crude, primitive and most of the gold is washed away back into the river. The current study mainly focused on extraction of placer gold deposits. Pneumatic machines from Passu to Shimshal (Hunza valley) Gilgit-Baltistan. The mercury amalgamation and cyanide leached methods have been used for placer gold deposits extraction from the concentrate obtained from shaking table. The amount of gold recovered from amalgamation method with mercury was 30.9%. The alloyed gold with other metals, gold dust, and fine gold was recovered by chemical process. The reagent consumption, i.e. 1.4 kg/ton of NACN and 6 Kg / ton of CaO were used for extraction of placer gold deposits. -

Horizons of CPEC in Gilgit-Baltistan. 6Th and 7Th August, Passu Hunza, Gilgit-Baltistan Conference Report
Negotiating Change for Sustainability: Horizons of CPEC in Gilgit-Baltistan. 6th and 7th August, Passu Hunza, Gilgit-Baltistan Conference Report Aga Khan Rural Support Programme Negotiating Change for Sustainability: Horizons of CPEC in Gilgit-Baltistan Aga Khan Rural Support Program (AKRSP) Baber Road, P.O. Box 506, Gilgit Gilgit-Baltistan, PaKistan Phone: (+92-5811) 52480/52910/52679 Fax: (+92-5811) 54175 Rapporteurs: Anees Abbas and Sumaira Editor: Aziz Ali Dad AKRSP@2017 1 | Page Contents ACKNOWLEDGEMENT ............................................................................................................................ 4 ACRONYMS ................................................................................................................................................ 5 EXCUTIVE SUMMARY ............................................................................................................................. 7 1. INTRODUCTION ............................................................................................................................... 10 1.1-OBJECTIVES ...................................................................................................................................... 10 1.2-STRUCTURE OF THE CONVENTION ............................................................................................. 10 2-INAUAGURAL SESSION ...................................................................................................................... 11 2.1 WELCOME SPEECH ................................................................................................................................ -

Role of Pakistan Studies in Promoting Political Awareness at Secondary Level in Pakistan
Bulletin of Education and Research December 2017, Vol. 39, No. 3 pp. 57-74 Role of Pakistan Studies in Promoting Political Awareness at Secondary Level in Pakistan Muhammad Muzaffar*, Muhammad Arshad Javaid** and Fariha Sohail*** _______________________________________________________________ Abstract This study was designed to determine the role of Pakistan studies in promoting political awareness among the secondary level students in Pakistan. The study was delimited to three divisions of the Punjab province. A sample of 480 students was taken from the High and Higher Secondary Schools of Faisalabad, Multan and Rawalpindi Divisions. The tools of research adopted in this study were content analysis of the curriculum of Pakistan Studies and a test regarding the basic political knowledge. The collected data were analyzed by using the descriptive as well as inferential statistics. The content analyses showed a little inclusion of the text on the basic political knowledge and the test results confirmed the lower level of political awareness among the students. The integration of the basic political information in the books of Pakistan studies at secondary level was recommended in this study. Keywords: Politics, political awareness, students, secondary school certificate, education, pakistan * Assistant Professor, Department of Political Science and International Relations, Government College University, Faisalabad. Email: [email protected] ** Lecturer University of Education Lahore, DG Khan Campus. *** Ph. D Scholar Department of Education, Government College University, Faisalabad. Role of Pakistan Studies in Promoting Political Awareness at SL in Pakistan 58 Introduction Pakistan is a country with a great multitude of population. “Even though the literacy rate is low, however the educational administration is constantly improving because of the concerted efforts on the part of successive governments. -

Passupassu Passu
NWFP Wildlife Department Government of NWFP NA Forestry, Parks and Wildlife Department NA Administration PASSUPASSUPASSUPASSU MagnificentPASSU Glaciers & Mighty Peaks Rock flutes near Fatima Hil © all photographs Matthieu Paley p12 PASSU IS MAGNIFICENTLY SITUATED, IN A COUNTRY WHERE NATURE HAS DESIGNED EVERYTHING ON A NOBLE AND LAVISH SCALE. “Col. R. C. F. Schomberg, “Between the Oxus and the Indus” ” all maps by Mareile Paley. Created with the help of WWF GIS-Lab, ESRI and ERDAS imagine all maps by Mareile Paley. PASSU FACTS location Gojal, Upper Hunza, on the Karakoram Highway main settlements Passu, Janabad public transport any transport on Karakoram Highway going between Aliabad and Sost accommodation several small guesthouses in Passu, guesthouses and hotels in Janabad supplies best to buy all your trekking food in Sost, Aliabad or Gilgit population 98 households, 880 people indigenous language Wakhi indigenous wildlife snow leopard, Himalayan ibex, red fox, wolf, Himalayan snow cock, rock partridge common plant species juniper, willow, poplar, wild roses, seabuckthorn View of Passu from Avdegar INTRODUCTION INTRODUCTIONINTRODUCTIONINTRODUCTIONINTRODUCTION With the completion of the Karakoram Highway (KKH) in 1978, the previously inaccessible maze of high mountains, glaciers and hidden valleys which make up the Karakoram Range, suddenly opened its doors to the world. An area of unspoiled mountain beauty with rugged glaciers, unclimbed peaks and pristine wildlife, Gojal, the region north of Hunza, has since become a popular spot for ecotourism. The sun-drenched summits Passu village with its small rural Wakhi community lays in the heart of Gojal. Passu’s idyllic Despite its popularity as a opposite Passu are commonly trekking destination, Passu known as the Cathedral Peaks. -

Custodians of Culture and Biodiversity
Custodians of culture and biodiversity Indigenous peoples take charge of their challenges and opportunities Anita Kelles-Viitanen for IFAD Funded by the IFAD Innovation Mainstreaming Initiative and the Government of Finland The opinions expressed in this manual are those of the authors and do not nec - essarily represent those of IFAD. The designations employed and the presenta - tion of material in this publication do not imply the expression of any opinion whatsoever on the part of IFAD concerning the legal status of any country, terri - tory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The designations “developed” and “developing” countries are in - tended for statistical convenience and do not necessarily express a judgement about the stage reached in the development process by a particular country or area. This manual contains draft material that has not been subject to formal re - view. It is circulated for review and to stimulate discussion and critical comment. The text has not been edited. On the cover, a detail from a Chinese painting from collections of Anita Kelles-Viitanen CUSTODIANS OF CULTURE AND BIODIVERSITY Indigenous peoples take charge of their challenges and opportunities Anita Kelles-Viitanen For IFAD Funded by the IFAD Innovation Mainstreaming Initiative and the Government of Finland Table of Contents Executive summary 1 I Objective of the study 2 II Results with recommendations 2 1. Introduction 2 2. Poverty 3 3. Livelihoods 3 4. Global warming 4 5. Land 5 6. Biodiversity and natural resource management 6 7. Indigenous Culture 7 8. Gender 8 9. -

Misuse of Licit Trade for Opiate Trafficking in Western and Central
MISUSE OF LICIT TRADE FOR OPIATE TRAFFICKING IN WESTERN AND CENTRAL ASIA MISUSE OF LICIT TRADE FOR OPIATE Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel: +(43) (1) 26060-0, Fax: +(43) (1) 26060-5866, www.unodc.org MISUSE OF LICIT TRADE FOR OPIATE TRAFFICKING IN WESTERN AND CENTRAL ASIA A Threat Assessment A Threat Assessment United Nations publication printed in Slovenia October 2012 MISUSE OF LICIT TRADE FOR OPIATE TRAFFICKING IN WESTERN AND CENTRAL ASIA Acknowledgements This report was prepared by the UNODC Afghan Opiate Trade Project of the Studies and Threat Analysis Section (STAS), Division for Policy Analysis and Public Affairs (DPA), within the framework of UNODC Trends Monitoring and Analysis Programme and with the collaboration of the UNODC Country Office in Afghanistan and in Pakistan and the UNODC Regional Office for Central Asia. UNODC is grateful to the national and international institutions that shared their knowledge and data with the report team including, in particular, the Afghan Border Police, the Counter Narcotics Police of Afghanistan, the Ministry of Counter Narcotics of Afghanistan, the customs offices of Afghanistan and Pakistan, the World Customs Office, the Central Asian Regional Information and Coordination Centre, the Customs Service of Tajikistan, the Drug Control Agency of Tajikistan and the State Service on Drug Control of Kyrgyzstan. Report Team Research and report preparation: Hakan Demirbüken (Programme management officer, Afghan Opiate Trade Project, STAS) Natascha Eichinger (Consultant) Platon Nozadze (Consultant) Hayder Mili (Research expert, Afghan Opiate Trade Project, STAS) Yekaterina Spassova (National research officer, Afghan Opiate Trade Project) Hamid Azizi (National research officer, Afghan Opiate Trade Project) Shaukat Ullah Khan (National research officer, Afghan Opiate Trade Project) A. -

A Case Study of Gilgit-Baltistan
The Role of Geography in Human Security: A Case Study of Gilgit-Baltistan PhD Thesis Submitted by Ehsan Mehmood Khan, PhD Scholar Regn. No. NDU-PCS/PhD-13/F-017 Supervisor Dr Muhammad Khan Department of Peace and Conflict Studies (PCS) Faculties of Contemporary Studies (FCS) National Defence University (NDU) Islamabad 2017 ii The Role of Geography in Human Security: A Case Study of Gilgit-Baltistan PhD Thesis Submitted by Ehsan Mehmood Khan, PhD Scholar Regn. No. NDU-PCS/PhD-13/F-017 Supervisor Dr Muhammad Khan This Dissertation is submitted to National Defence University, Islamabad in fulfilment for the degree of Doctor of Philosophy in Peace and Conflict Studies Department of Peace and Conflict Studies (PCS) Faculties of Contemporary Studies (FCS) National Defence University (NDU) Islamabad 2017 iii Thesis submitted in fulfilment of the requirement for Doctor of Philosophy in Peace and Conflict Studies (PCS) Peace and Conflict Studies (PCS) Department NATIONAL DEFENCE UNIVERSITY Islamabad- Pakistan 2017 iv CERTIFICATE OF COMPLETION It is certified that the dissertation titled “The Role of Geography in Human Security: A Case Study of Gilgit-Baltistan” written by Ehsan Mehmood Khan is based on original research and may be accepted towards the fulfilment of PhD Degree in Peace and Conflict Studies (PCS). ____________________ (Supervisor) ____________________ (External Examiner) Countersigned By ______________________ ____________________ (Controller of Examinations) (Head of the Department) v AUTHOR’S DECLARATION I hereby declare that this thesis titled “The Role of Geography in Human Security: A Case Study of Gilgit-Baltistan” is based on my own research work. Sources of information have been acknowledged and a reference list has been appended. -

Bilingual / Bi-Annual Pakistan Studies, English / Urdu Research Journal
I ISSN: 2311-6803 PAKISTAN STUDIES Bilingual / Bi-annual Pakistan Studies, English / Urdu Research Journal Vol. 03 Serial No. 1 January - June 2016 Editor: Dr.Mohammad Usman Tobawal PAKISTAN STUDY CENTER, UNIVERSITY OF BALOCHISTAN, QUETTA. II III MANAGING COMMITTE Patron Prof. Dr. Javaid Iqbal Vice Chancellor Editor Inchief Prof. Dr. Naheed Anjum Chishti Editor Dr. Muhammad Usman Tobawal Assistant Editors Dr. Noor Ahmed Prof. Dr. Kalimullah Prof. Dr. Ain ud Din Prof. Ghulam Farooq Baloch Prof. Yousuf Ali Rodeni Prof. Surriya Bano Associate Editors Prof. Taleem Badshah Mr. Qari Abdul Rehman Miss. Shazia Jaffar Mr. Nazir Ahmed Miss. Sharaf Bibi Composing Section Mr. Manzoor Ahmed Mr. Bijar Khan Mr. Pervaiz Ahmed IV EDITORIAL BOARD INTERNATIONAL Dr. Yanee Srimanee, Ministry of Commerce, Thailand. Prof. M. Aslam Syed Harvard University, Cambridge, Massachusetts. Dr. Jamil Farooqui Dept. of Sociology and Anthropology, International Islamic University, Kaula Lumpur Prof. Dr. Shinaz Jindani, Savannah State University of Georgia, USA Dr. Elina Bashir, University of Chicago. Dr. Murayama Kazuyuki, #26-106, Hamahata 5-10, Adachi-ku, Tokyo 1210061, Japan. Prof. Dr. Fida Muhammad, State University of New York Oneonta NY 12820 Dr. Naseer Dashti, 11 Sparows Lane, New Elthaw London, England SEQ2BP. Dr. Naseeb Ullah, International Correspondent, Editor & Political Consultant, The Montreal Tribune, Montreal, Quebec, Canada. Johnny Cheung Institute of Culture & Language Paris, France. V EDITORIAL BOARD NATIONAL Prof. Dr.Abdul Razzaq Sabir, Vice Chancellor, Turbat University. Dr. Fakhr-ul-Islam University of Peshawar. Dr. Abdul Saboor Pro Vice Chancellor, University of Turbat. Syed Minhaj ul Hassan, University of Peshawar. Prof. Dr. Javaid Haider Syed, Gujrat University.