Scientific Annual Report 2019
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polatuzumab Vedotin - R/R DLBCL RG7769 PD1-TIM3 Bimab - Solid Tumors NSCLC RG7446 Tecentriq – Her2-Pos
Pipeline summary Marketed products additional indications Global Development late-stage trials pRED (Roche Pharma Research & Early Development) gRED (Genentech Research & Early Development) 1 Changes to the development pipeline FY 2018 update New to phase I New to phase II New to phase III New to registration 3 NMEs: 2 NMEs transitioned from Ph1 1 NME transitioned from Ph2: 2 NMEs + 1 AI transitioned from Ph2 RG6217 - HBV RG7906 - psychiatric disorders RG6042 HTT ASO - Huntington’s following filing in EU and US: RG6237 - neuromuscular disorders RG6058 tiragolumab + Tecentriq - 6 AIs: RG7596 polatuzumab vedotin - r/r DLBCL RG7769 PD1-TIM3 biMAb - solid tumors NSCLC RG7446 Tecentriq – Her2-pos. BC neoadj RG6268 entrectinib - NSCLC ROS1+ 1 AI: 1 NME starting Ph2 RG7446 Tecentriq – high risk NMIBC RG6268 entrectinib – NTRK1 pan-tumor RG7601 Venclexta + gilteritinib – r/r RG6180 iNeST (personalized cancer RG6152 Xofluza - influenza hosp. patients 3 AIs transitioned from Ph3 following AML vaccine) + pembrolizumab - malignant RG6152 Xofluza – influenza pediatric filing in EU and US: melanoma patients RG7446 Tecentriq + nab-paclitaxel 1L 1 NME following termination of Ph3 RG7601 Venclexta - r/r MM t(11:14) non sq NSCLC RG7412 crenezumab – familial RG7601 Venclexta + HMA/LDAC - 1L RG7446 Tecentriq + nab-paclitaxel Alzheimer’s disease healthy individuals AML* 1LTNBC RG7446 Tecentriq + chemo - 1L extensive 1 AI: stage SCLC RG7446 Tecentriq SC – NSCLC Removed from phase I Removed from phase II Removed from phase III Removed from registration 2 NMEs: -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Tanibirumab (CUI C3490677) Add to Cart
5/17/2018 NCI Metathesaurus Contains Exact Match Begins With Name Code Property Relationship Source ALL Advanced Search NCIm Version: 201706 Version 2.8 (using LexEVS 6.5) Home | NCIt Hierarchy | Sources | Help Suggest changes to this concept Tanibirumab (CUI C3490677) Add to Cart Table of Contents Terms & Properties Synonym Details Relationships By Source Terms & Properties Concept Unique Identifier (CUI): C3490677 NCI Thesaurus Code: C102877 (see NCI Thesaurus info) Semantic Type: Immunologic Factor Semantic Type: Amino Acid, Peptide, or Protein Semantic Type: Pharmacologic Substance NCIt Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor tyrosine kinase expressed by endothelial cells, while VEGF is overexpressed in many tumors and is correlated to tumor progression. PDQ Definition: A fully human monoclonal antibody targeting the vascular endothelial growth factor receptor 2 (VEGFR2), with potential antiangiogenic activity. Upon administration, tanibirumab specifically binds to VEGFR2, thereby preventing the binding of its ligand VEGF. This may result in the inhibition of tumor angiogenesis and a decrease in tumor nutrient supply. VEGFR2 is a pro-angiogenic growth factor receptor -

Roche Template
Changes to the development pipeline Q3 2020 update New to phase I New to phase II New to phase III New to registration 7 NMEs: 2 NMEs: 4 NMEs: 1 NME: RG6091 UBE3A LNA - Angelman syndrome RG7992 FGFR1 x KLB - NASH RG6107 crovalimab - PNH RG6396 Gavreto (pralsetinib) - RET fusion- RG6286 NME - colorectal cancer CHU Oncolytic Type 5 adenovirus - esophageal RG6413+RG6412 REGN-COV2 - SARS-CoV-2 positive NSCLC RG6315 NME - systemic sclerosis cancer prophylaxis RG6330 KRAS G12C - KRAS-mutant solid tumors RG6171 SERD(3) ER+/HER2 - metastatic breast 2 AIs: RG6418 NLRP3 inhibitor - inflammation 5 AIs: cancer RG3648 Xolair - asthma home use 4D-MT 4D-R125 - X-linked retinitis pigmentosa RG6171 SERD(3) - neoadjuvant HR+ breast 7 AIs: RG6396 Gavreto (pralsetinitb) - RET-mutant CHU CD137 agonist switch antibody - solid tumors cancer RG6058 tiragolumab+Tecentriq - stage III medullary thyroid cancer (MTC) RG6413+RG6412 REGN-COV2 - COVID-19+ unresectable 1L NSCLC 2 AIs: adult – ambulatory RG6058 tiragolumab+Tecentriq - locally adv RG7440 ipatasertib+Tecentriq - prostate cancer RG6413+RG6412 REGN-COV2 - COVID-19+ esophageal cancer previously treated with androgen receptor-targeted adult – hospitalized RG6321 PDS with ranibizumab - diabetic therapy retinopathy RG7446 Tecentriq+Venclexta - maintenance 1L RG7159 Gazyva - lupus nephritis ES-SCLC RG7601 Venclexta - 1L MDS RG7446 Tecentriq+cabozantinib - adv RCC RG7446 Tecentriq+cabozantinib - 2L NSCLC Removed from phase I Removed from phase II Removed from phase III Approvals 2 NMEs: 2 AIs: 3 AIs: 3 NMEs approved -

The Angiopoietin-2 and TIE Pathway As a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer
International Journal of Molecular Sciences Review The Angiopoietin-2 and TIE Pathway as a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer Alessandra Leong and Minah Kim * Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY 10032, USA; afl[email protected] * Correspondence: [email protected] Received: 26 September 2020; Accepted: 13 November 2020; Published: 18 November 2020 Abstract: Despite significant advances made in cancer treatment, the development of therapeutic resistance to anticancer drugs represents a major clinical problem that limits treatment efficacy for cancer patients. Herein, we focus on the response and resistance to current antiangiogenic drugs and immunotherapies and describe potential strategies for improved treatment outcomes. Antiangiogenic treatments that mainly target vascular endothelial growth factor (VEGF) signaling have shown efficacy in many types of cancer. However, drug resistance, characterized by disease recurrence, has limited therapeutic success and thus increased our urgency to better understand the mechanism of resistance to inhibitors of VEGF signaling. Moreover, cancer immunotherapies including immune checkpoint inhibitors (ICIs), which stimulate antitumor immunity, have also demonstrated a remarkable clinical benefit in the treatment of many aggressive malignancies. Nevertheless, the emergence of resistance to immunotherapies associated with an immunosuppressive tumor microenvironment has restricted therapeutic response, necessitating the development of better therapeutic strategies to increase treatment efficacy in patients. Angiopoietin-2 (ANG2), which binds to the receptor tyrosine kinase TIE2 in endothelial cells, is a cooperative driver of angiogenesis and vascular destabilization along with VEGF. It has been suggested in multiple preclinical studies that ANG2-mediated vascular changes contribute to the development and persistence of resistance to anti-VEGF therapy. -

PD-1 / PD-L1 Combination Therapies
PD-1 / PD-L1 Combination Therapies Jacob Plieth & Edwin Elmhirst – May 2017 Foreword Sprinkling the immuno-oncology dust When in November 2015 EP Vantage published its first immuno- oncology analysis we identified 215 studies of anti-PD-1/PD-L1 projects combined with other approaches, and called this an important industry theme. It is a measure of how central combos have become that today, barely 18 months on, that total has been blown out of the water. No fewer than 765 studies involving combinations of PD-1 or PD-L1 assets are now listed on the Clinicaltrials.gov registry. This dazzling array owes much to the transformational nature of the data seen with the first wave of anti-PD-1/PD-L1 MAbs. It also indicates how central combinations will be in extending immuno-oncology beyond just a handful of cancers, and beyond certain patient subgroups. But the combo effort is as much about extending the reach of currently available drugs like Keytruda, Opdivo and Tecentriq as it is about making novel approaches viable by combining them with PD-1/PD-L1. On a standalone basis several of the industry’s novel oncology projects have underwhelmed. As data are generated it will therefore be vital for investors to tease out the effect of combinations beyond that of monotherapy, and it is doubtful whether the sprinkling of magic immuno-oncology dust will come to the rescue of substandard products. This is not stopping many companies, as the hundreds of combination studies identified here show. This report aims to quantify how many trials are ongoing with which assets and in which cancer indications, as well as suggesting reasons why some of the most popular approaches are being pursued. -

2017 Immuno-Oncology Medicines in Development
2017 Immuno-Oncology Medicines in Development Adoptive Cell Therapies Drug Name Organization Indication Development Phase ACTR087 + rituximab Unum Therapeutics B-cell lymphoma Phase I (antibody-coupled T-cell receptor Cambridge, MA www.unumrx.com immunotherapy + rituximab) AFP TCR Adaptimmune liver Phase I (T-cell receptor cell therapy) Philadelphia, PA www.adaptimmune.com anti-BCMA CAR-T cell therapy Juno Therapeutics multiple myeloma Phase I Seattle, WA www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 "armored" CAR-T Juno Therapeutics recurrent/relapsed chronic Phase I cell therapy Seattle, WA lymphocytic leukemia (CLL) www.junotherapeutics.com Memorial Sloan Kettering New York, NY anti-CD19 CAR-T cell therapy Intrexon B-cell malignancies Phase I Germantown, MD www.dna.com ZIOPHARM Oncology www.ziopharm.com Boston, MA anti-CD19 CAR-T cell therapy Kite Pharma hematological malignancies Phase I (second generation) Santa Monica, CA www.kitepharma.com National Cancer Institute Bethesda, MD Medicines in Development: Immuno-Oncology 1 Adoptive Cell Therapies Drug Name Organization Indication Development Phase anti-CEA CAR-T therapy Sorrento Therapeutics liver metastases Phase I San Diego, CA www.sorrentotherapeutics.com TNK Therapeutics San Diego, CA anti-PSMA CAR-T cell therapy TNK Therapeutics cancer Phase I San Diego, CA www.sorrentotherapeutics.com Sorrento Therapeutics San Diego, CA ATA520 Atara Biotherapeutics multiple myeloma, Phase I (WT1-specific T lymphocyte South San Francisco, CA plasma cell leukemia www.atarabio.com -

First-In-Human Phase I Study of Single-Agent Vanucizumab, a First-In
Author Manuscript Published OnlineFirst on December 7, 2017; DOI: 10.1158/1078-0432.CCR-17-1588 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Hidalgo et al. Clin Cancer Res submission 2017 First-in-human phase I study of single-agent vanucizumab, a first-in- class bi-specific anti-angiopoietin-2/anti-VEGF antibody, in adult patients with advanced solid tumors Manuel Hidalgo1,2*; Maria Martinez Garcia3*; Christophe Le Tourneau4; Christophe Massard5; Elena Garralda2; Valentina Boni2; Alvaro Taus3; Joan Albanell3; Marie-Paule Sablin4; Marie Alt4; Ratislav Bahleda5; Andrea Varga5; Christophe Boetsch6; Izolda Franjkovic7; Florian Heil7; Angelika Lahr7; Katharina Lechner7; Anthony Morel6; Tapan Nayak6; Simona Rossomanno6; Kevin Smart8; Kay Stubenrauch7; Oliver Krieter7. *Joint first authors 1Centro Nacional de Investigaciones Oncológicas (CNIO), Madrid, Spain. 2START Madrid-CIOCC, HM Sanchinarro.3Medical Oncology Department, Hospital del Mar. Barcelona, Spain.4Department of Medical Oncology, Institut Curie, Saint-Cloud & Paris.5Department of Drug Development, Gustave Roussy, Villejuif, France.6Roche Innovation Center Basel, Basel, Switzerland.7Roche Innovation Center Munich, Penzberg, Germany.8Roche Innovation Centre Welwyn, Welwyn Garden City, United Kingdom. Running title: Phase I study of vanucizumab Keywords: Vanucizumab, RG7221, RO5520985, VEGF-A, Ang-2 Corresponding authors: 1 Downloaded from clincancerres.aacrjournals.org on September 27, 2021. © 2017 American Association for Cancer Research. Author Manuscript Published OnlineFirst on December 7, 2017; DOI: 10.1158/1078-0432.CCR-17-1588 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Hidalgo et al. Clin Cancer Res submission 2017 Manuel Hidalgo, Chief, Division Hematology Oncology, Director, Rosenberg Clinical Cancer Center, Beth Israel Deaconess Medical Center, Member of the Faculty of Medicine, Harvard Medical School, 330 Brookline Avenue - KS207, Boston, MA 02215, USA. -

Gdzie Zginął „Antek Rozpylacz”
68 MIEJSCA Z HISTorią Gdzie zginął „Antek Rozpylacz” Katarzyna Dzierzbicka W Alejach Jerozolimskich w Warszawie, tuż obok sklepu „Vitkac”, wisi czarna tablica upamiętniająca „Antka Rozpylacza”. Wyryty na niej napis jest jednak mylący. Antoni Godlewski zginął około 300 metrów dalej. Miał 21 lat i nie był studentem medycyny. ntoni Szczęsny Godlew- rzony w 1944 roku. Szkoła Powszechna ski urodził się w Warsza- Towarzystwa Ziemi Mazowieckiej to wie 11 stycznia 1923 roku. dziś Gimnazjum i Liceum im. Narcy- Był synem Franciszka zy Żmichowskiej. W 1937 roku ojciec AGodlewskiego – wysokiego urzędni- przeniósł Antoniego do Zakładu Nauko- ka państwowego II RP, w latach 1927– wo-Wychowawczego Ojców Jezuitów –1933 wicewojewody nowogródzkiego, w Chyrowie koło Przemyśla, dziś leżą- 1934–1937 wicewojewody warszaw- cym na terenie Ukrainy. Askiego i 1937–1939 starosty powiatu warszawskiego. Po powrocie z Nowo- Łowca snajperów gródka Franciszek i Aniela Godlew- W czasie okupacji Antoni Godlew- Dzierzbicka Katarzyna Fot. scy wraz z synem ponownie zamiesz- ski studiował na Tajnej Politechnice kali w Warszawie, w al. Przyjaciół 3a. Warszawskiej. Omyłkowa informacja W 1942 roku rodzina została wyrzucona z tablicy pamiątkowej o studiach me- plutonowy podchorąży batalionu „So- z mieszkania przez Niemców. Państwo dycznych wzięła się najprawdopodob- kół”. Jego wspomnienia, jak również Godlewscy przenieśli się na ul. Mar- relacje pozostałych cytowanych prze- szałkowską 91 m. 19. Budynek ten zo- ze mnie kolegów „Antka Rozpylacza” stał zniszczony w czasie wojny. Dziś z czasów II wojny światowej, zostały w jego miejscu stoi biurowiec. Zbu- utrwalone na taśmach Archiwum Hi- dowane pod koniec lat trzydziestych storii Mówionej Muzeum Powstania XX wieku kamienice w al. Przyjaciół Warszawskiego. przetrwały za to wojenną zawieruchę. -

The Two Tontti Tudiul Lui Hi Ha Unit
THETWO TONTTI USTUDIUL 20170267753A1 LUI HI HA UNIT ( 19) United States (12 ) Patent Application Publication (10 ) Pub. No. : US 2017 /0267753 A1 Ehrenpreis (43 ) Pub . Date : Sep . 21 , 2017 ( 54 ) COMBINATION THERAPY FOR (52 ) U .S . CI. CO - ADMINISTRATION OF MONOCLONAL CPC .. .. CO7K 16 / 241 ( 2013 .01 ) ; A61K 39 / 3955 ANTIBODIES ( 2013 .01 ) ; A61K 31 /4706 ( 2013 .01 ) ; A61K 31 / 165 ( 2013 .01 ) ; CO7K 2317 /21 (2013 . 01 ) ; (71 ) Applicant: Eli D Ehrenpreis , Skokie , IL (US ) CO7K 2317/ 24 ( 2013. 01 ) ; A61K 2039/ 505 ( 2013 .01 ) (72 ) Inventor : Eli D Ehrenpreis, Skokie , IL (US ) (57 ) ABSTRACT Disclosed are methods for enhancing the efficacy of mono (21 ) Appl. No. : 15 /605 ,212 clonal antibody therapy , which entails co - administering a therapeutic monoclonal antibody , or a functional fragment (22 ) Filed : May 25 , 2017 thereof, and an effective amount of colchicine or hydroxy chloroquine , or a combination thereof, to a patient in need Related U . S . Application Data thereof . Also disclosed are methods of prolonging or increasing the time a monoclonal antibody remains in the (63 ) Continuation - in - part of application No . 14 / 947 , 193 , circulation of a patient, which entails co - administering a filed on Nov. 20 , 2015 . therapeutic monoclonal antibody , or a functional fragment ( 60 ) Provisional application No . 62/ 082, 682 , filed on Nov . of the monoclonal antibody , and an effective amount of 21 , 2014 . colchicine or hydroxychloroquine , or a combination thereof, to a patient in need thereof, wherein the time themonoclonal antibody remains in the circulation ( e . g . , blood serum ) of the Publication Classification patient is increased relative to the same regimen of admin (51 ) Int . -

See Who Attended
Company Name First Name Last Name Job Title Country 24Sea Gert De Sitter Owner Belgium 2EN S.A. George Droukas Data analyst Greece 2EN S.A. Yannis Panourgias Managing Director Greece 3E Geert Palmers CEO Belgium 3E Baris Adiloglu Technical Manager Belgium 3E David Schillebeeckx Wind Analyst Belgium 3E Grégoire Leroy Product Manager Wind Resource Modelling Belgium 3E Rogelio Avendaño Reyes Regional Manager Belgium 3E Luc Dewilde Senior Business Developer Belgium 3E Luis Ferreira Wind Consultant Belgium 3E Grégory Ignace Senior Wind Consultant Belgium 3E Romain Willaime Sales Manager Belgium 3E Santiago Estrada Sales Team Manager Belgium 3E Thomas De Vylder Marketing & Communication Manager Belgium 4C Offshore Ltd. Tom Russell Press Coordinator United Kingdom 4C Offshore Ltd. Lauren Anderson United Kingdom 4Cast GmbH & Co. KG Horst Bidiak Senior Product Manager Germany 4Subsea Berit Scharff VP Offshore Wind Norway 8.2 Consulting AG Bruno Allain Président / CEO Germany 8.2 Consulting AG Antoine Ancelin Commercial employee Germany 8.2 Monitoring GmbH Bernd Hoering Managing Director Germany A Word About Wind Zoe Wicker Client Services Manager United Kingdom A Word About Wind Richard Heap Editor-in-Chief United Kingdom AAGES Antonio Esteban Garmendia Director - Business Development Spain ABB Sofia Sauvageot Global Account Executive France ABB Jesús Illana Account Manager Spain ABB Miguel Angel Sanchis Ferri Senior Product Manager Spain ABB Antoni Carrera Group Account Manager Spain ABB Luis andres Arismendi Gomez Segment Marketing Manager Spain -
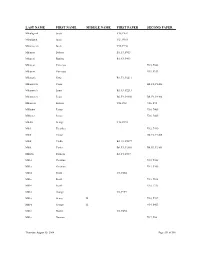
Last Name First Name Middle Name First Paper Second Paper
LAST NAME FIRST NAME MIDDLE NAME FIRST PAPER SECOND PAPER Mikoligczik Jacob V10, P452 Mikoligzik Jacob V21, P163 Mikotcyczik Jacob V10, P452 Milanesi Battista B1, F3, P413 Milanesi Battista B1, F3, P413 Milanesi Vincenzo V15, P866 Milanesi Vincenzo V11, P113 Milanovic Cazo B3, F3, P2213 Milanovich Caszo B6, F2, P2406 Milanovich Louis B3, F3, P2213 Milanovich Louis B6, F2, P2406 B6, F2, P2406 Milansesi Battista V26, P32 V26, P32 Milbouw Petrus V16, P465 Milbowv Petrus V16, P465 Milcki George V10, P293 Mild Theodore V12, P410 Milik Victor B6, F3, P2468 Milik Vinko B3, F3, P2277 Milik Vinko B6, F3, P2468 B6, F3, P2468 Miliotis Stamatis B2, F1, P237 Miller Christian V12, P342 Miller Christian V14, P365 Miller Frank V8, P204 Miller Frank V13, P636 Miller Frank V15, P725 Miller George V8, P139 Miller George H. V12, P397 Miller George H. V14, P423 Miller Martin V8, P494 Miller Norman V17, P86 Thursday, August 05, 2004 Page 351 of 568 LAST NAME FIRST NAME MIDDLE NAME FIRST PAPER SECOND PAPER Miller Rudolph Th. V12, P161 Miller Rudolph Th. V14, P175 Millie Mario V10, P186 Milligan Patrick V22, P195 V22, P195 Millman Alexander V14, P8 Millonig John V12, P435 Millonig John V14, P465 Millouw Petrus V10, P203 Milman Alexander V12, P5 Minapace Ignazia V12, P52 Minelli Filippo V9, P130 Minelli French V10, P130 Minelli Paele V9, P130 Minelli Pompilio V9, P124 Minenko Finarci B3, F3, P2291 Minenko Finarci B6, F3, P2494 Minenko Harry B3, F3, P2291 Minenko Harry B6, F3, P2494 B6, F3, P2494 Miners Edward Francis V25, P7 V25, P7 Miners George Alfred V26, P57