Parasitic Plants of the World Daniel L. Nickrent
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ethnomedicinal Aspects of Angiospermic Epiphytes and Parasites of Kerala, India
Indian Journal of Traditional Knowledge Vol. 11(2), April 2012, pp. 250-258 Ethnomedicinal aspects of angiospermic epiphytes and parasites of Kerala, India AE Shanavaskhan,1,2 M Sivadasan,3* Ahmed H Alfarhan,3 & Jacob Thomas3 1Tropical Botanic Garden & Research Institute, Palode P O, Thiruvananthapuram 695 562, Kerala, India 2Present address: Natural Resources and Environment Research Institute, King Abdulaziz City for Science and Technology, Riyadh, Kingdom of Saudi Arabia 3Department of Botany & Microbiology, College of Science, King Saud University, P O Box 2455, Riyadh 11451 Kingdom of Saudi Arabia *E-mail: [email protected] Received 15.07.2009; revised 10.03.2010 Studies on ethnomedicinal aspects of epiphytes and parasites of Kerala have been conducted and it revealed that as the tribes of Kerala have a lot of terrestrial medicinal plants available around their premises, they seldom resorted to the epiphytic and parasitic medicinal plants occurring on tall trees for their use as drugs for the treatment of ailments. Hence, their knowledge on epiphytes and parasites was found to be very limited, especially among the young generation of the tribes. The present study reported the use of 28 species (16 epiphytes and 12 parasites), which represent about 13.4% of the total epiphytes and parasites present in Kerala, and they are of valuable properties and uses and are used for curing or corrective measures for several diseases. Majority of the properties and uses recorded are first reports pertaining to these special groups of plants. A thorough investigation on the phytochemistry and therapeutic values of the bioactive compounds contained in these epiphytes and parasites would result in the discovery of new and valuable drugs of high potentials and of interest to the Nutraceutical and Pharmaceutical industries. -

A Checklist of the Vascular Flora of the Mary K. Oxley Nature Center, Tulsa County, Oklahoma
Oklahoma Native Plant Record 29 Volume 13, December 2013 A CHECKLIST OF THE VASCULAR FLORA OF THE MARY K. OXLEY NATURE CENTER, TULSA COUNTY, OKLAHOMA Amy K. Buthod Oklahoma Biological Survey Oklahoma Natural Heritage Inventory Robert Bebb Herbarium University of Oklahoma Norman, OK 73019-0575 (405) 325-4034 Email: [email protected] Keywords: flora, exotics, inventory ABSTRACT This paper reports the results of an inventory of the vascular flora of the Mary K. Oxley Nature Center in Tulsa, Oklahoma. A total of 342 taxa from 75 families and 237 genera were collected from four main vegetation types. The families Asteraceae and Poaceae were the largest, with 49 and 42 taxa, respectively. Fifty-eight exotic taxa were found, representing 17% of the total flora. Twelve taxa tracked by the Oklahoma Natural Heritage Inventory were present. INTRODUCTION clayey sediment (USDA Soil Conservation Service 1977). Climate is Subtropical The objective of this study was to Humid, and summers are humid and warm inventory the vascular plants of the Mary K. with a mean July temperature of 27.5° C Oxley Nature Center (ONC) and to prepare (81.5° F). Winters are mild and short with a a list and voucher specimens for Oxley mean January temperature of 1.5° C personnel to use in education and outreach. (34.7° F) (Trewartha 1968). Mean annual Located within the 1,165.0 ha (2878 ac) precipitation is 106.5 cm (41.929 in), with Mohawk Park in northwestern Tulsa most occurring in the spring and fall County (ONC headquarters located at (Oklahoma Climatological Survey 2013). -

Synthesizing Ecosystem Implications of Mistletoe Infection
Environmental Research Letters LETTER • OPEN ACCESS Related content - Networks on Networks: Water transport in Mistletoe, friend and foe: synthesizing ecosystem plants A G Hunt and S Manzoni implications of mistletoe infection - Networks on Networks: Edaphic constraints: the role of the soil in vegetation growth To cite this article: Anne Griebel et al 2017 Environ. Res. Lett. 12 115012 A G Hunt and S Manzoni - Impact of mountain pine beetle induced mortality on forest carbon and water fluxes David E Reed, Brent E Ewers and Elise Pendall View the article online for updates and enhancements. This content was downloaded from IP address 137.154.212.215 on 17/12/2017 at 21:57 Environ. Res. Lett. 12 (2017) 115012 https://doi.org/10.1088/1748-9326/aa8fff LETTER Mistletoe, friend and foe: synthesizing ecosystem OPEN ACCESS implications of mistletoe infection RECEIVED 28 June 2017 Anne Griebel1,3 ,DavidWatson2 and Elise Pendall1 REVISED 1 Hawkesbury Institute for the Environment, Western Sydney University, Locked Bag 1797, Penrith, NSW, Australia 12 September 2017 2 Institute for Land, Water and Society, Charles Sturt University, PO box 789, Albury, NSW, Australia ACCEPTED FOR PUBLICATION 3 Author to whom any correspondence should be addressed. 29 September 2017 PUBLISHED E-mail: [email protected] 16 November 2017 Keywords: mistletoe, climate change, biodiversity, parasitic plants, tree mortality, forest disturbance Original content from this work may be used Abstract under the terms of the Creative Commons Biotic disturbances are affecting a wide range of tree species in all climates, and their occurrence is Attribution 3.0 licence. contributing to increasing rates of tree mortality globally. -

Antiurolithiatic Plants: Multidimensional Pharmacology
Journal of Pharmacognosy and Phytochemistry 2016; 5(2): 04-24 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2016; 5(2): 04-24 Antiurolithiatic plants: Multidimensional Received: 04-01-2016 Accepted: 06-02-2016 pharmacology Salman Ahmed Lecturer, Department of Salman Ahmed, Muhammad Mohtasheemul Hasan, Zafar Alam Mahmood Pharmacognosy, Faculty of Pharmacy, University of Abstract Karachi, Karachi-75270, Urolithiasis is a common problem afflicted for many centuries with high recurrence. The aim of this Pakistan. review is to provide comprehensive information about traditionally used antiurolithiatic plants and their scientifically proved pharmacological activities like analgesic, anti-inflammatory, antioxidant, astringent, Muhammad Mohtasheemul Hasan demulcent, diuretic, litholytic, lithotriptic, antiurolithiatic, antispasmodic, ACE inhibition and Associate Professor, Department Phospholipase A2 inhibition as a plausible mechanism of action. A total of 503 species, 365 genera and of Pharmacognosy, Faculty of 119 families were cited for treating kidney stones. The most cited families are Asteraceae (41), Fabaceae Pharmacy, University of (34), Lamiaceae (26), Apiaceae (21), Rosaceae (19) and Poaceae (16). The most common used plant Karachi, Karachi-75270, parts are root and rhizome (25%), mode of preparation decoction (62%) and route of administration is Pakistan. oral in all cases. This review will provide the opportunities for the future research and development of new natural antiurolithiatic compounds. Zafar Alam Mahmood Colorcon Limited – UK, Keywords: urolithiasis, antiurolithiatic, natural products, drug development. Flagship House, Victory Way, Crossways, Dartford, Kent, DA26 QD- England. Introduction The belief and observations regarding traditionally used medicinal plants, increasing the interest of people to use natural medicine for their primary health care needs. A wide range of medicinal plants have been used in different countries and cultures as a prophylactic and curative agent for urolithiasis. -
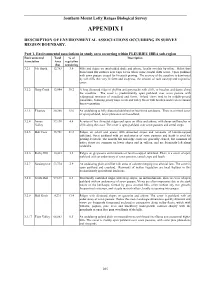
A Biological Survey of the Southern Mount Lofty Ranges
Southern Mount Lofty Ranges Biological Survey APPENDIX I DESCRIPTION OF ENVIRONMENTAL ASSOCIATIONS OCCURRING IN SURVEY REGION BOUNDARY. Part 1. Environmental associations in study area occurring within FLEURIEU IBRA sub-region Environmental Total % of Description Association Area vegetation (ha) remaining 3.2.1 Mt. Rapid 12,763 3.9 Hills and ridges on interbedded shale and arkose, locally overlain by tillite. Relict fans form broad flat surfaces near Cape Jervis where some coastal cliffs occur. Open parkland with sown pasture is used for livestock grazing. The scenery of the coastline is dominated by tall cliffs that vary in form and steepness, the amount of rock outcrop and vegetative cover. 3.2.2 Deep Creek 12,984 30.2 A long dissected ridge of phyllite and greywacke with cliffs, or beaches and dunes along the coastline. The cover is predominantly open parkland over sown pasture with widespread remnants of woodland and forest. Inland views tend to be middle-ground panoramic, featuring grassy ridge crests and valley floors with bracken and reed or remnant forest vegetation. 3.2.3 Fleurieu 30,389 15.6 An undulating to hilly dissected tableland on lateritized sandstone. There is a mixed cover of open parkland, forest plantation and woodland. 3.2.4 Inman 37,130 4.4 A series of low dissected ridges and spurs on tillite and arkose, with dunes and beaches or Valley cliffs along the coast. The cover is open parkland over sown pastures and cereal crops. 3.2.5 Bob Tiers 15,761 21.3 Ridges on schist and gneiss with dissected slopes and remnantsof laterite-capped tableland. -

The Genomic Impact of Mycoheterotrophy in Orchids
fpls-12-632033 June 8, 2021 Time: 12:45 # 1 ORIGINAL RESEARCH published: 09 June 2021 doi: 10.3389/fpls.2021.632033 The Genomic Impact of Mycoheterotrophy in Orchids Marcin J ˛akalski1, Julita Minasiewicz1, José Caius2,3, Michał May1, Marc-André Selosse1,4† and Etienne Delannoy2,3*† 1 Department of Plant Taxonomy and Nature Conservation, Faculty of Biology, University of Gdansk,´ Gdansk,´ Poland, 2 Institute of Plant Sciences Paris-Saclay, Université Paris-Saclay, CNRS, INRAE, Univ Evry, Orsay, France, 3 Université de Paris, CNRS, INRAE, Institute of Plant Sciences Paris-Saclay, Orsay, France, 4 Sorbonne Université, CNRS, EPHE, Muséum National d’Histoire Naturelle, Institut de Systématique, Evolution, Biodiversité, Paris, France Mycoheterotrophic plants have lost the ability to photosynthesize and obtain essential mineral and organic nutrients from associated soil fungi. Despite involving radical changes in life history traits and ecological requirements, the transition from autotrophy Edited by: Susann Wicke, to mycoheterotrophy has occurred independently in many major lineages of land Humboldt University of Berlin, plants, most frequently in Orchidaceae. Yet the molecular mechanisms underlying this Germany shift are still poorly understood. A comparison of the transcriptomes of Epipogium Reviewed by: Maria D. Logacheva, aphyllum and Neottia nidus-avis, two completely mycoheterotrophic orchids, to other Skolkovo Institute of Science autotrophic and mycoheterotrophic orchids showed the unexpected retention of several and Technology, Russia genes associated with photosynthetic activities. In addition to these selected retentions, Sean W. Graham, University of British Columbia, the analysis of their expression profiles showed that many orthologs had inverted Canada underground/aboveground expression ratios compared to autotrophic species. Fatty Craig Barrett, West Virginia University, United States acid and amino acid biosynthesis as well as primary cell wall metabolism were among *Correspondence: the pathways most impacted by this expression reprogramming. -

Pollen Evolution and Its Taxonomic Significance in Cuscuta (Dodders, Convolvulaceae)
Plant Syst Evol (2010) 285:83–101 DOI 10.1007/s00606-009-0259-4 ORIGINAL ARTICLE Pollen evolution and its taxonomic significance in Cuscuta (dodders, Convolvulaceae) Mark Welsh • Sasˇa Stefanovic´ • Mihai Costea Received: 12 June 2009 / Accepted: 28 December 2009 / Published online: 27 February 2010 Ó Springer-Verlag 2010 Abstract The pollen morphology of 148 taxa (135 spe- unknown in other Convolvulaceae, has evolved in Cuscuta cies and 13 varieties) of the parasitic plant genus Cuscuta only in two lineages (subg. Monogynella, and clade O of (dodders, Convolvulaceae) was examined using scanning subg. Grammica). Overall, the morphology of pollen electron microscopy. Six quantitative characters were supports Cuscuta as a sister to either the ‘‘bifid-style’’ coded using the gap-weighting method and optimized Convolvulaceae clade (Dicranostyloideae) or to one of the onto a consensus tree constructed from three large-scale members of this clade. Pollen characters alone are insuf- molecular phylogenies of the genus based on nuclear ficient to reconstruct phylogenetic relationships; however, internal transcribed spacer (ITS) and plastid trn-LF palynological information is useful for the species-level sequences. The results indicate that 3-zonocolpate pollen is taxonomy of Cuscuta. ancestral, while grains with more colpi (up to eight) have evolved only in two major lineages of Cuscuta (subg. Keywords Convolvulaceae Á Cuscuta Á Dodders Á Monogynella and clade O of subg. Grammica). Complex Evolution Á Phylogeny Á Pollen morphology Á morphological intergradations occur between species when Scanning electron microscopy Á Taxonomy their tectum is described using the traditional qualitative types—imperforate, perforate, and microreticulate. This continuous variation is better expressed quantitatively as Introduction ‘‘percent perforation,’’ namely the proportion of perforated area (puncta or lumina) from the total tectum surface. -

Physiological and Ecological Warnings That Dodder Pose an Exigent Threat
bioRxiv preprint doi: https://doi.org/10.1101/2020.10.26.355883; this version posted October 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Running title: The Exigent threat of Dodders in Eastern Africa 2 3 Title: Physiological and ecological warnings that Dodder pose an exigent threat to 4 farmlands in Eastern Africa 5 6 Joel Masanga1, Beatrice Njoki Mwangi1, Willy Kibet1, Philip Sagero2, Mark 7 Wamalwa1, Richard Oduor1, Mathew Ngugi1, Amos Alakonya3, Patroba Ojola1, 8 Emily S. Bellis4,5*, and Steven Runo1* 9 1 Department of Biochemistry, Microbiology and Biotechnology. Kenyatta University, Kenya. 10 2 Kenya Meteorological Department, Nairobi, Kenya 11 3 International Maize and Wheat Improvement Center, Mexico 12 4 Arkansas Biosciences Institute and Department of Computer Science, Arkansas State University, USA 13 5 Center for No-Boundary Thinking, Arkansas, USA 14 *Correspondence: [email protected] and [email protected] 15 16 Sentence Summary: Microscopy and habitat suitability modeling provide an early 17 warning that dodder’s invasion in Eastern Africa poses a threat to important cash crops 18 19 Funding information: We acknowledge financial support from Kenyatta University 20 through the Vice Chancellors Research grant number KU/DVCR/VRG/VOL.11/216. 21 JM’s PhD is funded by the National Research Fund (NRF) grant number 22 NRF/PhD/02/76. 23 24 Author contributions: S.R. conceived the study, guided fieldwork and oversaw 25 experimental work. -

The Vegetation of the Western Blue Mountains Including the Capertee, Coxs, Jenolan & Gurnang Areas
Department of Environment and Conservation (NSW) The Vegetation of the Western Blue Mountains including the Capertee, Coxs, Jenolan & Gurnang Areas Volume 1: Technical Report Hawkesbury-Nepean CMA CATCHMENT MANAGEMENT AUTHORITY The Vegetation of the Western Blue Mountains (including the Capertee, Cox’s, Jenolan and Gurnang Areas) Volume 1: Technical Report (Final V1.1) Project funded by the Hawkesbury – Nepean Catchment Management Authority Information and Assessment Section Metropolitan Branch Environmental Protection and Regulation Division Department of Environment and Conservation July 2006 ACKNOWLEDGMENTS This project has been completed by the Special thanks to: Information and Assessment Section, Metropolitan Branch. The numerous land owners including State Forests of NSW who allowed access to their Section Head, Information and Assessment properties. Julie Ravallion The Department of Natural Resources, Forests NSW and Hawkesbury – Nepean CMA for Coordinator, Bioregional Data Group comments on early drafts. Daniel Connolly This report should be referenced as follows: Vegetation Project Officer DEC (2006) The Vegetation of the Western Blue Mountains. Unpublished report funded by Greg Steenbeeke the Hawkesbury – Nepean Catchment Management Authority. Department of GIS, Data Management and Database Environment and Conservation, Hurstville. Coordination Peter Ewin Photos Kylie Madden Vegetation community profile photographs by Greg Steenbeeke Greg Steenbeeke unless otherwise noted. Feature cover photo by Greg Steenbeeke. All Logistics -

Maine Coefficient of Conservatism
Coefficient of Coefficient of Scientific Name Common Name Nativity Conservatism Wetness Abies balsamea balsam fir native 3 0 Abies concolor white fir non‐native 0 Abutilon theophrasti velvetleaf non‐native 0 3 Acalypha rhomboidea common threeseed mercury native 2 3 Acer ginnala Amur maple non‐native 0 Acer negundo boxelder non‐native 0 0 Acer pensylvanicum striped maple native 5 3 Acer platanoides Norway maple non‐native 0 5 Acer pseudoplatanus sycamore maple non‐native 0 Acer rubrum red maple native 2 0 Acer saccharinum silver maple native 6 ‐3 Acer saccharum sugar maple native 5 3 Acer spicatum mountain maple native 6 3 Acer x freemanii red maple x silver maple native 2 0 Achillea millefolium common yarrow non‐native 0 3 Achillea millefolium var. borealis common yarrow non‐native 0 3 Achillea millefolium var. millefolium common yarrow non‐native 0 3 Achillea millefolium var. occidentalis common yarrow non‐native 0 3 Achillea ptarmica sneezeweed non‐native 0 3 Acinos arvensis basil thyme non‐native 0 Aconitum napellus Venus' chariot non‐native 0 Acorus americanus sweetflag native 6 ‐5 Acorus calamus calamus native 6 ‐5 Actaea pachypoda white baneberry native 7 5 Actaea racemosa black baneberry non‐native 0 Actaea rubra red baneberry native 7 3 Actinidia arguta tara vine non‐native 0 Adiantum aleuticum Aleutian maidenhair native 9 3 Adiantum pedatum northern maidenhair native 8 3 Adlumia fungosa allegheny vine native 7 Aegopodium podagraria bishop's goutweed non‐native 0 0 Coefficient of Coefficient of Scientific Name Common Name Nativity -

Epiparasitism in Phoradendron Durangense and P. Falcatum (Viscaceae) Clyde L
Aliso: A Journal of Systematic and Evolutionary Botany Volume 27 | Issue 1 Article 2 2009 Epiparasitism in Phoradendron durangense and P. falcatum (Viscaceae) Clyde L. Calvin Rancho Santa Ana Botanic Garden, Claremont, California Carol A. Wilson Rancho Santa Ana Botanic Garden, Claremont, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Calvin, Clyde L. and Wilson, Carol A. (2009) "Epiparasitism in Phoradendron durangense and P. falcatum (Viscaceae)," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 27: Iss. 1, Article 2. Available at: http://scholarship.claremont.edu/aliso/vol27/iss1/2 Aliso, 27, pp. 1–12 ’ 2009, Rancho Santa Ana Botanic Garden EPIPARASITISM IN PHORADENDRON DURANGENSE AND P. FALCATUM (VISCACEAE) CLYDE L. CALVIN1 AND CAROL A. WILSON1,2 1Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711-3157, USA 2Corresponding author ([email protected]) ABSTRACT Phoradendron, the largest mistletoe genus in the New World, extends from temperate North America to temperate South America. Most species are parasitic on terrestrial hosts, but a few occur only, or primarily, on other species of Phoradendron. We examined relationships among two obligate epiparasites, P. durangense and P. falcatum, and their parasitic hosts. Fruit and seed of both epiparasites were small compared to those of their parasitic hosts. Seed of epiparasites was established on parasitic-host stems, leaves, and inflorescences. Shoots developed from the plumular region or from buds on the holdfast or subjacent tissue. The developing endophytic system initially consisted of multiple separate strands that widened, merged, and often entirely displaced its parasitic host from the cambial cylinder. -

Invisible Connections: Introduction to Parasitic Plants Dr
Invisible Connections: Introduction to Parasitic Plants Dr. Vanessa Beauchamp Towson University What is a parasite? • An organism that lives in or on an organism of another species (its host) and benefits by deriving nutrients at the other's expense. Symbiosis https://www.superpharmacy.com.au/blog/parasites-protozoa-worms-ectoparasites Food acquisition in plants: Autotrophy Heterotrophs (“different feeding”) • True parasites: obtain carbon compounds from host plants through haustoria. • Myco-heterotrophs: obtain carbon compounds from host plants via Image Credit: Flickr User wackybadger, via CC mycorrhizal fungal connection. • Carnivorous plants (not parasitic): obtain nutrients (phosphorus, https://commons.wikimedia.org/wiki/File:Pin nitrogen) from trapped insects. k_indian_pipes.jpg http://www.welivealot.com/venus-flytrap- facts-for-kids/ Parasite vs. Epiphyte https://chatham.ces.ncsu.edu/2014/12/does-mistletoe-harm-trees-2/ By © Hans Hillewaert /, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=6289695 True Parasitic Plants • Gains all or part of its nutrition from another plant (the host). • Does not contribute to the benefit of the host and, in some cases, causing extreme damage to the host. • Specialized peg-like root (haustorium) to penetrate host plants. https://www.britannica.com/plant/parasitic-plant https://chatham.ces.ncsu.edu/2014/12/does-mistletoe-harm-trees-2/ Diversity of parasitic plants Eudicots • Parasitism has evolved independently at least 12 times within the plant kingdom. • Approximately 4,500 parasitic species in Monocots 28 families. • Found in eudicots and basal angiosperms • 1% of the dicot angiosperm species • No monocot angiosperm species Basal angiosperms Annu. Rev. Plant Biol. 2016.67:643-667 True Parasitic Plants https://www.alamy.com/parasitic-dodder-plant-cuscuta-showing-penetration-parasitic-haustor The defining structural feature of a parasitic plant is the haustorium.