PHOTOSENSITIZING THIOPHENES from the "TAGETEAE By
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Coreopsideae Daniel J
Chapter42 Coreopsideae Daniel J. Crawford, Mes! n Tadesse, Mark E. Mort, "ebecca T. Kimball and Christopher P. "andle HISTORICAL OVERVIEW AND PHYLOGENY In a cladistic analysis of morphological features of Heliantheae by Karis (1993), Coreopsidinae were reported Morphological data to be an ingroup within Heliantheae s.l. The group was A synthesis and analysis of the systematic information on represented in the analysis by Isostigma, Chrysanthellum, tribe Heliantheae was provided by Stuessy (1977a) with Cosmos, and Coreopsis. In a subsequent paper (Karis and indications of “three main evolutionary lines” within "yding 1994), the treatment of Coreopsidinae was the the tribe. He recognized ! fteen subtribes and, of these, same as the one provided above except for the follow- Coreopsidinae along with Fitchiinae, are considered ing: Diodontium, which was placed in synonymy with as constituting the third and smallest natural grouping Glossocardia by "obinson (1981), was reinstated following within the tribe. Coreopsidinae, including 31 genera, the work of Veldkamp and Kre# er (1991), who also rele- were divided into seven informal groups. Turner and gated Glossogyne and Guerreroia as synonyms of Glossocardia, Powell (1977), in the same work, proposed the new tribe but raised Glossogyne sect. Trionicinia to generic rank; Coreopsideae Turner & Powell but did not describe it. Eryngiophyllum was placed as a synonym of Chrysanthellum Their basis for the new tribe appears to be ! nding a suit- following the work of Turner (1988); Fitchia, which was able place for subtribe Jaumeinae. They suggested that the placed in Fitchiinae by "obinson (1981), was returned previously recognized genera of Jaumeinae ( Jaumea and to Coreopsidinae; Guardiola was left as an unassigned Venegasia) could be related to Coreopsidinae or to some Heliantheae; Guizotia and Staurochlamys were placed in members of Senecioneae. -

ASTERACEAE José Ángel Villarreal-Quintanilla* José Luis Villaseñor-Ríos** Rosalinda Medina-Lemos**
FLORA DEL VALLE DE TEHUACÁN-CUICATLÁN Fascículo 62. ASTERACEAE José Ángel Villarreal-Quintanilla* José Luis Villaseñor-Ríos** Rosalinda Medina-Lemos** *Departamento de Botánica Universidad Autónoma Agraria Antonio Narro **Departamento de Botánica Instituto de Biología, UNAM INSTITUTO DE BIOLOGÍA UNIVERSIDAD NACIONAL AUTÓNOMA DE MÉXICO 2008 Primera edición: octubre de 2008 D.R. © Universidad Nacional Autónoma de México Instituto de Biología. Departamento de Botánica ISBN 968-36-3108-8 Flora del Valle de Tehuacán-Cuicatlán ISBN 970-32-5084-4 Fascículo 62 Dirección de los autores: Departamento de Botánica Universidad Autónoma Agraria Antonio Narro Buenavista, Saltillo C.P. 25315 Coahuila, México Universidad Nacional Autónoma de México Instituto de Biología. Departamento de Botánica. 3er. Circuito de Ciudad Universitaria Coyoacán, 04510. México, D.F. 1 En la portada: 2 1. Mitrocereus fulviceps (cardón) 2. Beaucarnea purpusii (soyate) 3 4 3. Agave peacockii (maguey fibroso) 4. Agave stricta (gallinita) Dibujo de Elvia Esparza FLORA DEL VALLE DE TEHUACÁN-CUICATLÁN 62: 1-59. 2008 ASTERACEAE1 Bercht. & J.Presl Tribu Tageteae José Ángel Villarreal-Quintanilla José Luis Villaseñor-Ríos Rosalinda Medina-Lemos Bibliografía. Bremer, K. 1994. Asteraceae. Cladistics & Classification. Timber Press. Portland, Oregon. 752 p. McVaugh, R. 1984. Compositae. In: W.R. Anderson (ed.). Flora Novo-Galiciana. Ann Arbor The University of Michi- gan Press 12: 40-42. Panero, J.L. & V.A. Funk. 2002. Toward a phylogene- tic subfamily classification for the Compositae (Asteraceae). Proc. Biol. Soc. Washington 115: 909-922. Villaseñor Ríos, J.L. 1993. La familia Asteraceae en México. Rev. Soc. Mex. Hist. Nat. 44: 117-124. Villaseñor Ríos, J.L. 2003. Diversidad y distribución de las Magnoliophyta de México. -

Chromosome Numbers in Compositae, XII: Heliantheae
SMITHSONIAN CONTRIBUTIONS TO BOTANY 0 NCTMBER 52 Chromosome Numbers in Compositae, XII: Heliantheae Harold Robinson, A. Michael Powell, Robert M. King, andJames F. Weedin SMITHSONIAN INSTITUTION PRESS City of Washington 1981 ABSTRACT Robinson, Harold, A. Michael Powell, Robert M. King, and James F. Weedin. Chromosome Numbers in Compositae, XII: Heliantheae. Smithsonian Contri- butions to Botany, number 52, 28 pages, 3 tables, 1981.-Chromosome reports are provided for 145 populations, including first reports for 33 species and three genera, Garcilassa, Riencourtia, and Helianthopsis. Chromosome numbers are arranged according to Robinson’s recently broadened concept of the Heliantheae, with citations for 212 of the ca. 265 genera and 32 of the 35 subtribes. Diverse elements, including the Ambrosieae, typical Heliantheae, most Helenieae, the Tegeteae, and genera such as Arnica from the Senecioneae, are seen to share a specialized cytological history involving polyploid ancestry. The authors disagree with one another regarding the point at which such polyploidy occurred and on whether subtribes lacking higher numbers, such as the Galinsoginae, share the polyploid ancestry. Numerous examples of aneuploid decrease, secondary polyploidy, and some secondary aneuploid decreases are cited. The Marshalliinae are considered remote from other subtribes and close to the Inuleae. Evidence from related tribes favors an ultimate base of X = 10 for the Heliantheae and at least the subfamily As teroideae. OFFICIALPUBLICATION DATE is handstamped in a limited number of initial copies and is recorded in the Institution’s annual report, Smithsonian Year. SERIESCOVER DESIGN: Leaf clearing from the katsura tree Cercidiphyllumjaponicum Siebold and Zuccarini. Library of Congress Cataloging in Publication Data Main entry under title: Chromosome numbers in Compositae, XII. -
1 B 2 C 3 B 4 D 5 D
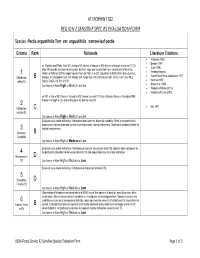
ATTACHMENT SS2 REGION 2 SENSITIVE SPECIES EVALUATION FORM Species: Pectis angustifolia Torr. var. angustifolia narrow-leaf pectis Criteria Rank Rationale Literature Citations • Anderson 1950 • Barkley 1991 se (Goshen and Platte Cos) WY, e into w NE; thence s through w KS; thence w through e and sw (?) CO. • Clark 1996 Most NE records are from the sw corner, but Keil maps one record from the n-central part of the state. 1 Weber & Wittman (2001b) report species from La Plata in w CO. [Vouchers at KANU from Baca, Lincoln, • Freeman in prep. • Great Plains Flora Association 1977 Distribution B Morgan, and Sedgwick Cos, CO; Meade and Trego Cos, KS; and Deuel, Keith, and Lincoln Cos, NE.] within R2 Status: G4G5; KS SH; WY S1 • Hartman 1997 • Shaw et al. 1989 Confidence in Rank High or Medium or Low • Weber & Wittman 2001a • Weber & Witman 2001b se WY, e into w NE; thence s through w KS; thence s w-most TX into n Mexico; thence n throughout NM; thence n through e CO, and w through n AZ and se-most UT. 2 • Keil 1977 Distribution C outside R2 Confidence in Rank High or Medium or Low Evaluator was unable to find any information about species’ dispersal capability. Seed is equipped with a pappus and may be dispersed by wind or perhaps water, during heavy rains. Dispersal is probably limited by 3 habitat requirements. Dispersal B Capability Confidence in Rank High or Medium or Low Evaluator was unable to find any information on species’ abundance within R2. Species does not appear to 4 be particularly abundant where encountered in CO and populations may face local extinction. -

Ndhf Sequence Evolution and the Major Clades in the Sunflower Family KI-JOONG KIM* and ROBERT K
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 10379-10383, October 1995 Evolution ndhF sequence evolution and the major clades in the sunflower family KI-JOONG KIM* AND ROBERT K. JANSENt Department of Botany, University of Texas, Austin, TX 78713-7640 Communicated by Peter H. Raven, Missouri Botanical Garden, St. Louis, MO, June 21, 1995 ABSTRACT An extensive sequence comparison of the either too short or too conserved to provide adequate numbers chloroplast ndhF gene from all major clades of the largest of characters in recently evolved families. A number of alter- flowering plant family (Asteraceae) shows that this gene native genes have been suggested as potential candidates for provides -3 times more phylogenetic information than rbcL. phylogenetic comparisons at lower taxonomic levels (9). The This is because it is substantially longer and evolves twice as phylogenetic utility of one of these, matK, has been recently fast. The 5' region (1380 bp) ofndhF is very different from the demonstrated (10). Comparison of sequences of two chloro- 3' region (855 bp) and is similar to rbcL in both the rate and plast genomes (rice and tobacco), however, revealed only two the pattern of sequence change. The 3' region is more A+T- genes, rpoCl and ndhF, that are considerably longer and evolve rich, has higher levels of nonsynonymous base substitution, faster than rbcL (9, 11). We selected ndhF because it is longer and shows greater transversion bias at all codon positions. and evolves slightly faster than rpoCl (11), because rpoCl has These differences probably reflect different functional con- an intron that may require additional effort in DNA amplifi- straints on the 5' and 3' regions of nduhF. -
![ASHY DOGWEED (Thymophylla [=Dyssodia] Tephroleuca)](https://docslib.b-cdn.net/cover/9459/ashy-dogweed-thymophylla-dyssodia-tephroleuca-729459.webp)
ASHY DOGWEED (Thymophylla [=Dyssodia] Tephroleuca)
ASHY DOGWEED (Thymophylla [=Dyssodia] tephroleuca) 5-Year Review: Summary and Evaluation Photograph: Chris Best, USFWS U.S. Fish and Wildlife Service Corpus Christi Ecological Services Field Office Corpus Christi, Texas September 2011 1 FIVE YEAR REVIEW Ashy dogweed/Thymophylla tephroleuca Blake 1.0 GENERAL INFORMATION 1.1 Reviewers Lead Regional Office: Southwest Regional Office, Region 2 Susan Jacobsen, Chief, Threatened and Endangered Species, 505-248-6641 Wendy Brown, Endangered Species Recovery Coordinator, 505-248-6664 Julie McIntyre, Recovery Biologist, 505-248-6507 Lead Field Office: Corpus Christi Ecological Services Field Office Robyn Cobb, Fish and Wildlife Biologist, 361- 994-9005, ext. 241 Amber Miller, Fish and Wildlife Biologist, 361-994-9005, ext. 247 Cooperating Field Office: Austin Ecological Services Field Office Chris Best, Texas State Botanist, 512- 490-0057, ext. 225 1.2 Purpose of 5-Year Reviews: The U.S. Fish and Wildlife Service (Service or USFWS) is required by section 4(c)(2) of the Endangered Species Act (Act) to conduct a status review of each listed species once every five years. The purpose of a 5-year review is to evaluate whether or not the species’ status has changed since it was listed (or since the most recent 5-year review). Based on the 5-year review, we recommend whether the species should be removed from the list of endangered and threatened species, be changed in status from endangered to threatened, or be changed in status from threatened to endangered. Our original listing as endangered or threatened is based on the species’ status considering the five threat factors described in section 4(a)(1) of the Act. -

Vascular Plants and a Brief History of the Kiowa and Rita Blanca National Grasslands
United States Department of Agriculture Vascular Plants and a Brief Forest Service Rocky Mountain History of the Kiowa and Rita Research Station General Technical Report Blanca National Grasslands RMRS-GTR-233 December 2009 Donald L. Hazlett, Michael H. Schiebout, and Paulette L. Ford Hazlett, Donald L.; Schiebout, Michael H.; and Ford, Paulette L. 2009. Vascular plants and a brief history of the Kiowa and Rita Blanca National Grasslands. Gen. Tech. Rep. RMRS- GTR-233. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 44 p. Abstract Administered by the USDA Forest Service, the Kiowa and Rita Blanca National Grasslands occupy 230,000 acres of public land extending from northeastern New Mexico into the panhandles of Oklahoma and Texas. A mosaic of topographic features including canyons, plateaus, rolling grasslands and outcrops supports a diverse flora. Eight hundred twenty six (826) species of vascular plant species representing 81 plant families are known to occur on or near these public lands. This report includes a history of the area; ethnobotanical information; an introductory overview of the area including its climate, geology, vegetation, habitats, fauna, and ecological history; and a plant survey and information about the rare, poisonous, and exotic species from the area. A vascular plant checklist of 816 vascular plant taxa in the appendix includes scientific and common names, habitat types, and general distribution data for each species. This list is based on extensive plant collections and available herbarium collections. Authors Donald L. Hazlett is an ethnobotanist, Director of New World Plants and People consulting, and a research associate at the Denver Botanic Gardens, Denver, CO. -

Lectotypification of Pectis L. (Asteraceae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DigitalCommons@CalPoly LECTOTYPIFICATION OF PECTIS L. (ASTERACEAE) David J. Keil'Kei/ t Linnaeus (I(1759a)759a) described Pectis and included within it two species, P. ciliaris and P. linifolia. In 1913 Britton and Brown designated P. ciliaris as the lectotype for Pectis. This lectotypification was accepted by the editors of Index Nominum Genericorum (Plantarum) (FaIT(Farr et aI.,al., 1979). I will present evidence below that the lectotypification of Pectis by Britton and Brown was superfluous and that Pectis was actually lectotypified over 90 years earlier by Cassini. Britton and Brown as adherents of the American Code of Botanical Nomenclature (Anonymous, 1907) followed the mechanical practice of designating "the first binomial species in order" as the lectotype of a genus. This appears to have been the method of lectotypification used for Pectis. This practice is in conflict with the provisions of Article 8 of the International Code of Botanical NomenNomen- clature (Voss et aI.,al., 1983). The choice of Pectis ciliaris as lectotype is also in conflict with the Linnaean protologue of Pectis. Linnaeus (I(1759a)759a) included both a diagnosis (p. 1221) and a description (p. 1376) for Pectis. The diagnosis is very brief: PECTIS. Recept. nudum. Pappus aristatus. Cal. 5-phyllus, cylindricus. Flosculi radiantes 5. Of the features described, the receptacle, the involucreinvolucre [caL][cal.] and the number of ray florets are the same for both P. ciliaris and P. linifolia.linifolia. However, only P. linifolialinifolia has an aristate pappus. -

SAN DIEGO COUNTY NATIVE PLANTS in the 1830S
SAN DIEGO COUNTY NATIVE PLANTS IN THE 1830s The Collections of Thomas Coulter, Thomas Nuttall, and H.M.S. Sulphur with George Barclay and Richard Hinds James Lightner San Diego Flora San Diego, California 2013 SAN DIEGO COUNTY NATIVE PLANTS IN THE 1830s Preface The Collections of Thomas Coulter, Thomas Nuttall, and Our knowledge of the natural environment of the San Diego region H.M.S. Sulphur with George Barclay and Richard Hinds in the first half of the 19th century is understandably vague. Referenc- es in historical sources are limited and anecdotal. As prosperity peaked Copyright © 2013 James Lightner around 1830, probably no more than 200 inhabitants in the region could read and write. At most one or two were trained in natural sciences or All rights reserved medicine. The best insights we have into the landscape come from nar- No part of this document may be reproduced or transmitted in any form ratives of travelers and the periodic reports of the missions’ lands. They without permission in writing from the publisher. provide some idea of the extent of agriculture and the general vegeta- tion covering surrounding land. ISBN: 978-0-9749981-4-5 The stories of the visits of United Kingdom naturalists who came in Library of Congress Control Number: 2013907489 the 1830s illuminate the subject. They were educated men who came to the territory intentionally to examine the flora. They took notes and col- Cover photograph: lected specimens as botanists do today. Reviewing their contributions Matilija Poppy (Romneya trichocalyx), Barrett Lake, San Diego County now, we can imagine what they saw as they discovered plants we know. -

Essential Oil of Dyssodia Acerosa DC
3276 J. Agric. Food Chem. 1997, 45, 3276−3278 Essential Oil of Dyssodia acerosa DC. Mario R. Tellez,* Rick E. Estell, Ed L. Fredrickson, and Kris M. Havstad Jornada Experimental Range, Agricultural Research Service, U.S. Department of Agriculture, P.O. Box 30003, MSC 3JER, New Mexico State University, Las Cruces, New Mexico 88003 Samples of the suffrutescent Dyssodia acerosa DC. were collected from the Jornada Experimental Range in south-central New Mexico, and GC/MS and retention indices were used to identify 62 components from the steam-distilled oil. Chrysanthenone (34.8%), limonene (14.5%), camphor (12.3%), R-pinene (6.8%), sabinene (4.3%), camphene (3.9%), (E)-â-ocimene (2.5%), 1,8-cineole (2.2%), myrcene (2.0%), and cis-sabinene hydrate (1.6%) were the major components of the steam-distilled oil. Keywords: Dyssodia acerosa; Asteraceae; essential oil composition; chrysanthenone; limonene; camphor INTRODUCTION leorthid soils (Tencee series) formed on an old alluvial fan (Neher and Bailey, 1976; Gibbens et al., 1993). Long-term The study of diet selection by livestock in arid annual precipitation (1922-1990) at the nearest rain gauge rangelands has been a long-term research focus at the (1.7 kM from the collection site; elevation of 1585 m) is 257 U.S. Department of Agriculture, Agricultural Research mm, with 55% of the precipitation occurring in July, August, Service’s Jornada Experimental Range (JER). Recently, and September (Gibbens et al., 1993). Total precipitation we have begun to examine plant-herbivore interactions between October 1995 and September 1996 was 215 mm (73 at the biochemical level (Estell et al., 1994; Fredrickson mm in July, 20 mm in August, and 60 mm in September). -

Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B
Aliso: A Journal of Systematic and Evolutionary Botany Volume 29 | Issue 1 Article 4 2011 Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B. Harper Terra Peninsular, Coronado, California Sula Vanderplank Rancho Santa Ana Botanic Garden, Claremont, California Mark Dodero Recon Environmental Inc., San Diego, California Sergio Mata Terra Peninsular, Coronado, California Jorge Ochoa Long Beach City College, Long Beach, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Biodiversity Commons, Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Harper, Alan B.; Vanderplank, Sula; Dodero, Mark; Mata, Sergio; and Ochoa, Jorge (2011) "Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 29: Iss. 1, Article 4. Available at: http://scholarship.claremont.edu/aliso/vol29/iss1/4 Aliso, 29(1), pp. 25–42 ’ 2011, Rancho Santa Ana Botanic Garden PLANTS OF THE COLONET REGION, BAJA CALIFORNIA, MEXICO, AND A VEGETATION MAPOF COLONET MESA ALAN B. HARPER,1 SULA VANDERPLANK,2 MARK DODERO,3 SERGIO MATA,1 AND JORGE OCHOA4 1Terra Peninsular, A.C., PMB 189003, Suite 88, Coronado, California 92178, USA ([email protected]); 2Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711, USA; 3Recon Environmental Inc., 1927 Fifth Avenue, San Diego, California 92101, USA; 4Long Beach City College, 1305 East Pacific Coast Highway, Long Beach, California 90806, USA ABSTRACT The Colonet region is located at the southern end of the California Floristic Province, in an area known to have the highest plant diversity in Baja California. -

Porophyllum Genus Compounds and Pharmacological Activities: a Review
Scientia Pharmaceutica Review Porophyllum Genus Compounds and Pharmacological Activities: A Review María José Vázquez-Atanacio 1,2 , Mirandeli Bautista-Ávila 2,* , Claudia Velázquez-González 2, Araceli Castañeda-Ovando 3 , Manasés González-Cortazar 4, Carolina Guadalupe Sosa-Gutiérrez 1 and Deyanira Ojeda-Ramírez 1,* 1 Área Académica de Medicina Veterinaria y Zootecnia, Instituto de Ciencias Agropecuarias, Universidad Autónoma del Estado de Hidalgo, Av. Universidad Km 1, Ex-Hda. de Aquetzalpa, Tulancingo 43600, Mexico; [email protected] (M.J.V.-A.); [email protected] (C.G.S.-G.) 2 Área Académica de Farmacia, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Ex Hacienda la Concepción s/n, San Agustín Tlaxiaca 42160, Mexico; [email protected] 3 Área Académica de Química de Alimentos, Instituto de Ciencias Básicas e Ingenierías, Universidad Autónoma del Estado de Hidalgo, Pachuca-Tulancingo km 4.5 Carboneras, Pachuca de Soto 42184, Mexico; [email protected] 4 Centro de Investigación Biomédica del Sur, Instituto Mexicano del Seguro Social, Argentina No. 1., Centro, Xochitepec 62790, Mexico; [email protected] * Correspondence: [email protected] (M.B.-Á.); [email protected] (D.O.-R.) Abstract: The genus Porophyllum (family Asteraceae) is native to the western hemisphere, growing in tropical and subtropical North and South America. Mexico is an important center of diversification of the genus. Plants belong of genus Porophyllum have been used in Mexican traditional medicine to treat kidney and intestinal diseases, parasitic, bacterial, and fungal infections and anti-inflammatory and anti-nociceptive activities. In this sense, several trials have been made on its chemical and in vitro Citation: Vázquez-Atanacio, M.J.; and in vivo pharmacological activities.