Invasion Dynamics of a Non-Indigenous Bivalve, Nuttallia Obscurata, (Reeve 1857), in the Northeast Pacific
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Manifest Destiny – Clam Style by Bert Bartleson “Manifest Destiny” Was One of the Concepts Taught to Me During American History in High School
Manifest Destiny – Clam Style By Bert Bartleson “Manifest Destiny” was one of the concepts taught to me during American History in high school. It was the inevitability of American settlers during the nineteenth century marching across the entire continent to the Pacific Ocean. In the world of introduced mollusk species, it seems that some successful invaders will expand their territory until they run out of unoccupied space or reach some physical barrier, much like the settlers reaching the Pacific Ocean. The “purple varnish clam [PVC] or Purple Mahogany-clam”, Nuttallia obscurata (Reeve, 1847) seems to be one of these very successful introduced species. They are native in Japan, Korea and possibly China. They usually live quite high in the intertidal zone so don’t directly compete for food with the native littlenecks, Manila clams, butter clams and horse clams that all prefer lower tidal levels on our local beaches. It is likely that they were released with ballast water near Vancouver, B.C., Canada waters about 1990. They were first observed during August 1991 by Robert Forsythe. A report was published in 1993 in the Dredgings. The clams spread southward to Boundary Bay and Birch Bay in Whatcom County and into the San Juan Islands quite quickly. They also spread rapidly to the North in the Strait of Georgia. The reason for this rapid spread wasn’t immediately known. But in 2006 a paper by Dudas and Dower helped to explain why. They studied the larval period that the veliger stage of the clam was still in the Bert Bartleson photo water column. -

Shellfish/Seaweed Rules
Washington Sport Fishing Rules: Effective May 1, 2014 - June 30, 2015 Shellfish/Seaweed Rules for current beach seasons check http://wdfw.wa.gov/fishing/shellfish/beaches/, New: Shellfish Rule Change Hotline (866) 880-5431 or contact the WdFW customer service desk (360) 902-2700 to verify seasons. 3 Steps to Safe and Legal Shellfish Harvest - It's your responsibility! 1 � � � � Know the Rules (You could get a ticket) Is the harvesting season open? Read the rules for seasons, size, and bag limits. For beach seasons, check the website http://wdfw.wa.gov/fishing/shellfish/beaches/, the toll-free WdFW Shellfish Rule Change Hotline (866) 880-5431, or contact the WdFW customer service desk (360) 902-2700 to verify seasons. 2 � � � � Pollution Closures (You could get sick) Does the beach meet standards for healthy eating? Some closures are shown on the map on page 128. For more pollution closures visit the Washington department of Health website at www.doh.wa.gov/shellfishsafety.htm, call (360) 236-3330 or the local county health department. 3 � � � � Marine Biotoxin Closures andVibrio Warnings (You could get sick or die) Is there an emergency closure due to Shellfish Poisoning (PSP/ASP/DSP) or Vibrio bacteria? Check the dOH website at www.doh.wa.gov/shellfishsafety.htm, call (360) 236-3330, or the Shellfish Safety toll-free Hotline at (800) 562-5632. Licenses Possession Limit Safe Handling A Combination or a Shellfish/Seaweed License one daily limit in fresh form. Additional Practices is required for all shellfish (except CRaWFISH) shellfish may be possessed in frozen or and SEaWEEd harvest. -

West Coast Shellfish Research Goals -- 2015 Priorities Table of Contents Executive Summary
West Coast Shellfish Research Goals -- 2015 Priorities Table of Contents Executive Summary..................................................................................................... 1 West Coast Goals and Priorities Background ............................................................... 1 Topic 1: Protection & Enhancement of Water Quality in Shellfish Growing Areas ......... 2 Goal 1.1. Respond to water quality problems in a coordinated and constructive manner. ......... 2 Goal 1.2. Develop a strategy for engaging the community in the health of local waters and galvanizing broader community support for pollution control projects. ..................................... 2 Topic 2: Shellfish Health, Disease Prevention and Management .................................. 3 Goal 2.1. Develop disease prevention, surveillance and treatment strategies to ensure the production of high health shellfish which meet domestic and international health standards and maximize production efficiencies. ......................................................................................... 3 Goal 2.2. Conduct research to enhance understanding of the extent and impact of ocean acidification on shellfish and identify and develop potential response and management strategies. ..................................................................................................................................... 4 Topic 3: Shellfish Ecology ............................................................................................ 4 Goal 3.1. Promote the -

Black Oystercatcher Foraging Hollenberg and Demers 35
Black Oystercatcher foraging Hollenberg and Demers 35 Black Oystercatcher (Haematopus bachmani) foraging on varnish clams (Nuttallia obscurata) in Nanaimo, British Columbia Emily J. R. Hollenberg 1 and Eric Demers 2 1 4063905 Quadra St., Victoria, B.C., V8X 1J1; email: [email protected] 2 Corresponding author: Biology Department, Vancouver Island University, 900 Fifth St., Nanaimo, B.C., V9R 5S5; email: [email protected] Abstract: In this study, we investigated whether Black Oystercatchers (Haematopus bachmani) feed on the recently intro duced varnish clam (Nuttallia obscurata), and whether they selectively feed on specific size classes of varnish clams. Sur veys were conducted at Piper’s Lagoon and Departure Bay in Nanaimo, British Columbia, between October 2013 and February 2014. Foraging oystercatchers were observed, and the number and size of varnish clams consumed were recor ded. We also determined the density and size of varnish clams available at both sites using quadrats. Our results indicate that Black Oystercatchers consumed varnish clams at both sites, although feeding rates differed slightly between sites. We also found that oystercatchers consumed almost the full range of available clam sizes, with little evidence for sizeselective foraging. We conclude that Black Oystercatchers can successfully exploit varnish clams and may obtain a significant part of their daily energy requirements from this nonnative species. Key Words: Black Oystercatcher, Haematopus bachmani, varnish clam, Nuttallia obscurata, foraging, Nanaimo. Hollenberg, E.J.R. and E. Demers. 2017. Black Oystercatcher (Haematopus bachmani) foraging on varnish clams (Nuttallia obscurata) in Nanaimo, British Columbia. British Columbia Birds 27:35–41. Campbell et al. -

Physiological Adaptations and Feeding Mechanisms of the Invasive Purple Varnish Clam, Nuttallia Obscurata
Western Washington University Western CEDAR WWU Graduate School Collection WWU Graduate and Undergraduate Scholarship 2013 Physiological adaptations and feeding mechanisms of the invasive purple varnish clam, Nuttallia obscurata Leesa E. Sorber Western Washington University Follow this and additional works at: https://cedar.wwu.edu/wwuet Part of the Biology Commons Recommended Citation Sorber, Leesa E., "Physiological adaptations and feeding mechanisms of the invasive purple varnish clam, Nuttallia obscurata" (2013). WWU Graduate School Collection. 281. https://cedar.wwu.edu/wwuet/281 This Masters Thesis is brought to you for free and open access by the WWU Graduate and Undergraduate Scholarship at Western CEDAR. It has been accepted for inclusion in WWU Graduate School Collection by an authorized administrator of Western CEDAR. For more information, please contact [email protected]. PHYSIOLOGICAL ADAPTATIONS AND FEEDING MECHANISMS OF THE INVASIVE PURPLE VARNISH CLAM, NUTTALLIA OBSCURATA by Leesa E. Sorber Accepted in Partial Completion of the Requirements for the Degree Master of Science Kathleen L. Kitto, Dean of the Graduate School ADVISORY COMMITTEE Chair, Dr. Deborah Donovan Dr. Benjamin Miner Dr. Jose Serrano-Moreno MASTER’S THESIS In presenting this thesis in partial fulfillment of the requirements for a master’s degree at Western Washington University, I grant Western Washington University the non-exclusive royalty-free right to archive, reproduce, distribute, and display the thesis in any and all forms, including electronic format, via any digital library mechanisms maintained by WWU. I represent and warrant this is my original work, and does not infringe or violate any rights of others. I warrant that I have obtained written permission for the owner of any third party copyrighted material included in these files. -

List of Bivalve Molluscs from British Columbia, Canada
List of Bivalve Molluscs from British Columbia, Canada Compiled by Robert G. Forsyth Research Associate, Invertebrate Zoology, Royal BC Museum, 675 Belleville Street, Victoria, BC V8W 9W2; [email protected] Rick M. Harbo Research Associate, Invertebrate Zoology, Royal BC Museum, 675 Belleville Street, Victoria BC V8W 9W2; [email protected] Last revised: 11 October 2013 INTRODUCTION Classification rankings are constantly under debate and review. The higher classification utilized here follows Bieler et al. (2010). Another useful resource is the online World Register of Marine Species (WoRMS; Gofas 2013) where the traditional ranking of Pteriomorphia, Palaeoheterodonta and Heterodonta as subclasses is used. This list includes 237 bivalve species from marine and freshwater habitats of British Columbia, Canada. Marine species (206) are mostly derived from Coan et al. (2000) and Carlton (2007). Freshwater species (31) are from Clarke (1981). Common names of marine bivalves are from Coan et al. (2000), who adopted most names from Turgeon et al. (1998); common names of freshwater species are from Turgeon et al. (1998). Changes to names or additions to the fauna since these two publications are marked with footnotes. Marine groups are in black type, freshwater taxa are in blue. Introduced (non-indigenous) species are marked with an asterisk (*). Marine intertidal species (n=84) are noted with a dagger (†). Quayle (1960) published a BC Provincial Museum handbook, The Intertidal Bivalves of British Columbia. Harbo (1997; 2011) provided illustrations and descriptions of many of the bivalves found in British Columbia, including an identification guide for bivalve siphons and “shows”. Lamb & Hanby (2005) also illustrated many species. -

Responses of Aquatic Non-Native Species to Novel Predator Cues and Increased Mortality
Portland State University PDXScholar Dissertations and Theses Dissertations and Theses Spring 5-17-2017 Responses of Aquatic Non-Native Species to Novel Predator Cues and Increased Mortality Brian Christopher Turner Portland State University Follow this and additional works at: https://pdxscholar.library.pdx.edu/open_access_etds Part of the Terrestrial and Aquatic Ecology Commons Let us know how access to this document benefits ou.y Recommended Citation Turner, Brian Christopher, "Responses of Aquatic Non-Native Species to Novel Predator Cues and Increased Mortality" (2017). Dissertations and Theses. Paper 3620. https://doi.org/10.15760/etd.5512 This Dissertation is brought to you for free and open access. It has been accepted for inclusion in Dissertations and Theses by an authorized administrator of PDXScholar. Please contact us if we can make this document more accessible: [email protected]. Responses of Aquatic Non-Native Species to Novel Predator Cues and Increased Mortality by Brian Christopher Turner A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Environmental Sciences and Resources Dissertation Committee: Catherine E. de Rivera, Chair Edwin D. Grosholz Michael T. Murphy Greg M. Ruiz Ian R. Waite Portland State University 2017 Abstract Lethal biotic interactions strongly influence the potential for aquatic non-native species to establish and endure in habitats to which they are introduced. Predators in the recipient area, including native and previously established non-native predators, can prevent establishment, limit habitat use, and reduce abundance of non-native species. Management efforts by humans using methods designed to cause mass mortality (e.g., trapping, biocide applications) can reduce or eradicate non-native populations. -
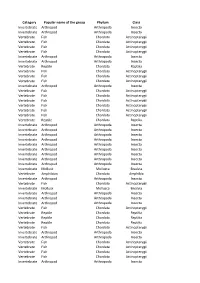
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Catalog of Recent and Fossil Molluscan Types in the Santa Barbara Museum of Natural History. I. Caudofoveata
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/256082238 Catalog of Recent and Fossil Molluscan Types in the Santa Barbara Museum of Natural History. I. Caudofoveata... Article in Veliger -Berkeley- · January 1990 CITATIONS READS 4 108 3 authors: Paul Valentich-Scott F.G. Hochberg Santa Barbara Museum of Natural History Santa Barbara Museum of Natural History 66 PUBLICATIONS 537 CITATIONS 48 PUBLICATIONS 755 CITATIONS SEE PROFILE SEE PROFILE Barry Roth 176 PUBLICATIONS 1,113 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Marine Bivalve Mollusks of Western South America View project Description of new polygyrid land snails from Oregon and California View project Available from: Paul Valentich-Scott Retrieved on: 21 November 2016 THE VELIGER © CMS, Inc., 1990 The Veliger 33(Suppl. 1):1-27 (January 2, 1990) Catalog of Recent and Fossil Molluscan Types in the Santa Barbara Museum of Natural History. I. Caudofoveata, Polyplacophora, Bivalvia, Scaphopoda, and Cephalopoda by PAUL H. SCOTT, F. G. HOCHBERG, AND BARRY ROTH Department of Invertebrate Zoology, Santa Barbara Museum of Natural History, 2559 Puesta del Sol, Santa Barbara, California 93105, USA Abstract. The non-gastropod molluscan types currently housed in the Department of Invertebrate Zoology at the Santa Barbara Museum are listed. Three hundred seventeen type lots are reported, representing 211 recent species and 9 species originally described as fossils. Each type lot recorded includes a complete citation, type locality, and the current type status of the specimens. An author index and alphabetic index are provided. -

An Annotated Checklist of the Marine Macroinvertebrates of Alaska David T
NOAA Professional Paper NMFS 19 An annotated checklist of the marine macroinvertebrates of Alaska David T. Drumm • Katherine P. Maslenikov Robert Van Syoc • James W. Orr • Robert R. Lauth Duane E. Stevenson • Theodore W. Pietsch November 2016 U.S. Department of Commerce NOAA Professional Penny Pritzker Secretary of Commerce National Oceanic Papers NMFS and Atmospheric Administration Kathryn D. Sullivan Scientific Editor* Administrator Richard Langton National Marine National Marine Fisheries Service Fisheries Service Northeast Fisheries Science Center Maine Field Station Eileen Sobeck 17 Godfrey Drive, Suite 1 Assistant Administrator Orono, Maine 04473 for Fisheries Associate Editor Kathryn Dennis National Marine Fisheries Service Office of Science and Technology Economics and Social Analysis Division 1845 Wasp Blvd., Bldg. 178 Honolulu, Hawaii 96818 Managing Editor Shelley Arenas National Marine Fisheries Service Scientific Publications Office 7600 Sand Point Way NE Seattle, Washington 98115 Editorial Committee Ann C. Matarese National Marine Fisheries Service James W. Orr National Marine Fisheries Service The NOAA Professional Paper NMFS (ISSN 1931-4590) series is pub- lished by the Scientific Publications Of- *Bruce Mundy (PIFSC) was Scientific Editor during the fice, National Marine Fisheries Service, scientific editing and preparation of this report. NOAA, 7600 Sand Point Way NE, Seattle, WA 98115. The Secretary of Commerce has The NOAA Professional Paper NMFS series carries peer-reviewed, lengthy original determined that the publication of research reports, taxonomic keys, species synopses, flora and fauna studies, and data- this series is necessary in the transac- intensive reports on investigations in fishery science, engineering, and economics. tion of the public business required by law of this Department. -

The Mode of Life in the Genus Pholadomya As Inferred from the Fossil Record
geosciences Article The Mode of Life in the Genus Pholadomya as Inferred from the Fossil Record Przemysław Sztajner Institute of Marine and Environmental Sciences, University of Szczecin, Mickiewicza 16A, 70-383 Szczecin, Poland; [email protected] Received: 13 July 2020; Accepted: 21 September 2020; Published: 5 October 2020 Abstract: The paper is an attempt to reconstruct the mode of life of Pholadomya bivalves, very common in the fossil record, particularly that of the Jurassic. The only extant representative of the genus is extremely rare and very poorly known. Materials from the Polish Jurassic deposits (Bajocian–Kimmeridgian; Western Pomerania and Polish Jura) and literature data were used for the reconstruction. Specifically, observations on the anatomy, taphonomy, and diagenesis of the specimens examined as well as lithology of the deposits housing the specimens were used. Shell anatomy characteristics are known for their particular utility in mode of life reconstructions, although the extremely thin-shelled and coarsely sculpted bivalves, such as the Pholadomya examined, have not been studied so far. The reconstruction suggest a diversity of the mode of life, coincident with the morphological differences between the Pholadomya species. At least the adults of anteriorly flattened species are inferred to have lived extremely deeply buried in the sediment, and were hardly mobile. The smaller, more oval in shape, species were more mobile, and some of them are thought to have preferred life in shelters, should those be available. In addition, the function of the cruciform muscle, other than that considered so far, is suggested. Keywords: Pholadomya; Anomalodesmata; Bivalvia; life habit; deep burrowers; taphonomy; shell anatomy; cruciform muscle; Jurassic; Poland 1. -

Of Mitochondria: Investigating the Functioning, Maintenance and Evolutionary Relevance of a Naturally Heteroplasmic System
Université de Montréal Energy metabolism in species with Doubly Uniparental Inheritance (DUI) of mitochondria: investigating the functioning, maintenance and evolutionary relevance of a naturally heteroplasmic system Par Stefano Bettinazzi Département de sciences biologiques, Faculté des arts et des sciences Thèse présentée en vue de l’obtention du grade de Philosophiae Doctor (Ph.D.) en sciences biologiques June 2020 © Stefano Bettinazzi, 2020 A été évaluée par un jury composé des personnes suivantes Sandra Binning Président-rapporteur Sophie Breton Directeur de recherche Pierre Blier Codirecteur Annie Anger Membre du jury Ron Burton Examinateur externe RÉSUMÉ Les mitochondries et leur génome, l'ADN mitochondrial (ADNmt), sont généralement transmis uniquement par la mère aux fils et aux filles chez les métazoaires (transmission strictement maternelle, SMI). Une exception à la règle générale de la SMI se trouve dans environ 100 espèces de bivalves, qui se caractérise par une double transmission uniparentale (DUI) des mitochondries. Chez les espèces DUI, deux lignées d'ADNmt très divergentes et liées au sexe coexistent. Une lignée mitochondriale maternelle (type F), présente dans les ovocytes et les tissus somatiques des individus femelles et males, et une lignée paternelle (type M), présente dans les spermatozoïdes. Dans les tissus somatiques mâles, les deux lignées coexistent parfois, une condition appelée hétéroplasmie. En sachant que les variations génétiques dans l’ADNmt peuvent avoir un impact sur les fonctions mitochondriales, et en donnant l'association stricte des ADNmt de type M et F avec différents gamètes, il est imaginable que la forte divergence entre les deux lignées DUI puisse entraîner des adaptations bioénergétiques avec répercussion sur la reproduction.