Tissue Variability in the Infaunal Bivalve Axinopsida Serricata
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
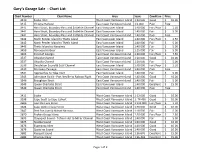
Gary's Charts
Gary’s Garage Sale - Chart List Chart Number Chart Name Area Scale Condition Price 3410 Sooke Inlet West Coast Vancouver Island 1:20 000 Good $ 10.00 3415 Victoria Harbour East Coast Vancouver Island 1:6 000 Poor Free 3441 Haro Strait, Boundary Pass and Sattelite Channel East Vancouver Island 1:40 000 Fair/Poor $ 2.50 3441 Haro Strait, Boundary Pass and Sattelite Channel East Vancouver Island 1:40 000 Fair $ 5.00 3441 Haro Strait, Boundary Pass and Sattelite Channel East Coast Vancouver Island 1:40 000 Poor Free 3442 North Pender Island to Thetis Island East Vancouver Island 1:40 000 Fair/Poor $ 2.50 3442 North Pender Island to Thetis Island East Vancouver Island 1:40 000 Fair $ 5.00 3443 Thetis Island to Nanaimo East Vancouver Island 1:40 000 Fair $ 5.00 3459 Nanoose Harbour East Vancouver Island 1:15 000 Fair $ 5.00 3463 Strait of Georgia East Coast Vancouver Island 1:40 000 Fair/Poor $ 7.50 3537 Okisollo Channel East Coast Vancouver Island 1:20 000 Good $ 10.00 3537 Okisollo Channel East Coast Vancouver Island 1:20 000 Fair $ 5.00 3538 Desolation Sound & Sutil Channel East Vancouver Island 1:40 000 Fair/Poor $ 2.50 3539 Discovery Passage East Coast Vancouver Island 1:40 000 Poor Free 3541 Approaches to Toba Inlet East Vancouver Island 1:40 000 Fair $ 5.00 3545 Johnstone Strait - Port Neville to Robson Bight East Coast Vancouver Island 1:40 000 Good $ 10.00 3546 Broughton Strait East Coast Vancouver Island 1:40 000 Fair $ 5.00 3549 Queen Charlotte Strait East Vancouver Island 1:40 000 Excellent $ 15.00 3549 Queen Charlotte Strait East -

British Columbia Regional Guide Cat
National Marine Weather Guide British Columbia Regional Guide Cat. No. En56-240/3-2015E-PDF 978-1-100-25953-6 Terms of Usage Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: • Exercise due diligence in ensuring the accuracy of the materials reproduced; • Indicate both the complete title of the materials reproduced, as well as the author organization; and • Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the author. For more information, please contact Environment Canada’s Inquiry Centre at 1-800-668-6767 (in Canada only) or 819-997-2800 or email to [email protected]. Disclaimer: Her Majesty is not responsible for the accuracy or completeness of the information contained in the reproduced material. Her Majesty shall at all times be indemnified and held harmless against any and all claims whatsoever arising out of negligence or other fault in the use of the information contained in this publication or product. Photo credits Cover Left: Chris Gibbons Cover Center: Chris Gibbons Cover Right: Ed Goski Page I: Ed Goski Page II: top left - Chris Gibbons, top right - Matt MacDonald, bottom - André Besson Page VI: Chris Gibbons Page 1: Chris Gibbons Page 5: Lisa West Page 8: Matt MacDonald Page 13: André Besson Page 15: Chris Gibbons Page 42: Lisa West Page 49: Chris Gibbons Page 119: Lisa West Page 138: Matt MacDonald Page 142: Matt MacDonald Acknowledgments Without the works of Owen Lange, this chapter would not have been possible. -

PART 3 Scale 1: Publication Edition 46 W Puget Sound – Point Partridge to Point No Point 50,000 Aug
Natural Date of New Chart No. Title of Chart or Plan PART 3 Scale 1: Publication Edition 46 w Puget Sound – Point Partridge to Point No Point 50,000 Aug. 1995 July 2005 Port Townsend 25,000 47 w Puget Sound – Point No Point to Alki Point 50,000 Mar. 1996 Sept. 2003 Everett 12,500 48 w Puget Sound – Alki Point to Point Defi ance 50,000 Dec. 1995 Aug. 2011 A Tacoma 15,000 B Continuation of A 15,000 50 w Puget Sound – Seattle Harbor 10,000 Mar. 1995 June 2001 Q1 Continuation of Duwamish Waterway 10,000 51 w Puget Sound – Point Defi ance to Olympia 80,000 Mar. 1998 - A Budd Inlet 20,000 B Olympia (continuation of A) 20,000 80 w Rosario Strait 50,000 Mar. 1995 June 2011 1717w Ports in Juan de Fuca Strait - July 1993 July 2007 Neah Bay 10,000 Port Angeles 10,000 1947w Admiralty Inlet and Puget Sound 139,000 Oct. 1893 Sept. 2003 2531w Cape Mendocino to Vancouver Island 1,020,000 Apr. 1884 June 1978 2940w Cape Disappointment to Cape Flattery 200,000 Apr. 1948 Feb. 2003 3125w Grays Harbor 40,000 July 1949 Aug. 1998 A Continuation of Chehalis River 40,000 4920w Juan de Fuca Strait to / à Dixon Entrance 1,250,000 Mar. 2005 - 4921w Queen Charlotte Sound to / à Dixon Entrance 525,000 Oct. 2008 - 4922w Vancouver Island / Île de Vancouver-Juan de Fuca Strait to / à Queen 525,000 Mar. 2005 - Charlotte Sound 4923w Queen Charlotte Sound 365,100 Mar. -

The British Columbia Coast 1967
SHRIMP EXPLORATION ON THE BRITISH COLUMBIA COAST 1967 BY M.S.SMITH AND T.H.BUTLER FISHERIES RESEARCH BOARD OF CANADA BIOLOGICAL STATION , NANAIMO B.C. CIRCULAR NO.85 FEBRUARY 1968 ~ < SHRIMP EXPLORATION ON THE BRITISH COLUMBIA COAST 1967 BY M.S.SMITH AND T.H.BUTLER FISHERIES RESEARCH BOARD OF CANADA BIOLOGICAL STATION , NANAI MO B.C. CIRCULAR NO.85 FEBRUARY 1968 INTRODUCTION The Fisheries Research Board of Canada, with the aid of funds provided by the Industrial Development Service of the Department of Fisheries, con- f . tinued to carry out exploration for new shrimp grounds in 1967. Two observers ^ from the Biological Station at Nanaimo, British Columbia, were aboard a ^ . chartered vessel from July 4 to September 9, 1967. The objective of the survey was to find concentrations of shrimp on the outer continental shelf of British Columbia. The programme, consisted of studying areas considered to have commercial potential and re-assessing the potential of grounds found in past shrimp explorations. VESSEL AND EXTENT OF SURVEY The commercial trawler M.V. Ocean Traveller, under Captain Frank Gale, was chartered to carry out the programme. The vessel is a conventional wooden-hulled combination vessel of 80 gross tons (63 ft keel length) powered by a 220 hp diesel engine. Deck equipment included a hydraulic powered combination seine-trawl winch and a hydraulic net reel, conven tionally rigged for double gear trawling* Each winch drum carried approximately 400 fathoms of 9/16" galvanized wire. Auxiliary equipment included an Ekolite 9B sounder, radar, loran and radios. (This was the same captain and vessel that conducted the Industrial Development Service exploratory groundfish surveys in.1965 and 1966). -

Department Of· Fisheries of Canada Vancouver, B. C
DEPARTMENT OF· FISHERIES OF CANADA VANCOUVER, B. C. 1968 This booklet lists the names and shows the locat·ions of all main stem salmon spawning streams in British Columbia, exclusive of those streams draining through Southeastern Alaska. Not all tributary streams have been included in the listing. I I This material represents a portion of the information being . ' collected for the preparation of an inventory of salmon bearing streams in the Pacific Region. PREPARED BY RESOURCE DEVELOPMENT BRANCH IN COLLABORATION ·WITH CONSERVATION & PROTECTION BRANCH Edited by C. E. Walker DEPARTMENT OF FISHERIES OF CANADA PACIFIC AREA MAP SHOWING PROTECTION DISTRICTS AND STATISTICAL ,l\.REAS '- ·-" " . ~--L~-t--?.>~1> ,j '\ "·, -;:.~ '-, ~ .., -" '.) \ 'Uppe_r Arrow Loire \ ) \ ' ('ZC:t;I ;-Koafenoy ;:Lower (!~ LoJ<e Cranb~~"\ \Arrow ',\ ·• ·~ ·\. 1 i 1.AP NU. P. DIS1 • STA'rI3TICAL lAREAS LOCA'rION ..... ··-· ..... -~ ...... ... ~- ............... .. - . ................. ~ .. - ····-·~ --· ·---' --~ .. -'•··--·--·---- .. ·--""'· .. ..._..-~ ...-- ....... ..~---·-··-.-·- ... ---·· l 1 Sub-~District Cari boo ') 1 Sub-District Prince GeorGe ') .) 1 3ub-·-DJ.strict Kamloops.--Lj_llooet· 2 ~issioti-Harrison: Chilli.'wa ck--HoyJe Lower Fraser River ~~ 28 & 29 Howe Sound: New Jestminster 6 3 17, 18, 19 & 20 Nanaimo, Duncan, Victoria c.: 'Port San Juan 7 3 l~· Comox 8 3 15 Toba Inlet (~estview) () ,/ 3 16 Pender Harbour 10 Li- 22 & 23 Nitinat & Barkley Sound 11 Li- 24 Clayoquot Sound 12 l+ 25 Nootka Sound 13 l+ 26 Kyuquot Sound 14 5 J.l Seymour - Belize 15 5 12 Alert Bay (Broughton) 16 5 12 Alert Bay (Knight Inlet) , 1 ..... 17 5 --J Campbell River .., () ..L ~) 5 27 Quatsino Sound 6 9 &·10 Rivers Inlet & Smith Inlet ,..., ,.. 20 ( 0 Butedale (Fraser I\each) 21 '7 6 Butedale (Ki tima t Ar::.1) ') ') l.-t·- '7 7 Bella Bella r'"J ( 8 Bella Coola 8 3 Nass .. -

Quatsino Sound Coastal Plan
QQuuaattssiinnoo SSoouunndd Coastal Plan Coastal Plan 30 March 2004 Ministry of Fisheries and Oceans Pêches et Ocèans Sustainable Resource Management Canada Canada Coast & Marine Planning Branch National Library of Canada Cataloguing in Publication Data British Columbia. Coast and Marine Planning Branch. Quatsino Sound Coastal Plan Also available on the Internet. Includes bibliographical references: p. ISBN 0-7726-4912-X 1. Coastal zone management – Government policy - British Columbia – Quatsino Sound Region. 2. Land use - British Columbia – Quatsino Sound Region – Planning. I. Title. HD319.B7B74 2003 333.91’7’097112 C2003-960035-1 Purpose This policy document has been developed cooperatively by the Coast and Marine Planning Branch, BC Ministry of Sustainable Resource Management and the Central Coast Regional Office of Fisheries and Oceans Canada. It is intended to provide direction for provincial tenure approval in the intertidal and nearshore environment of the Plan Area and policy guidance for Fisheries and Oceans staff within the Quatsino Coastal Management Area. Letter from the Regional Director General of Fisheries and Oceans Canada Acknowledgements The Quatsino Sound Coastal Plan was shaped by the advice and kind support of many individuals. Special thanks are extended to the Regional District of Mount Waddington for assisting the Ministry with public and technical input to the Plan, particularly the tireless efforts of Annemarie Koch, and Bill Shephard. The Plan could not have been successfully prepared without the participation of the Quatsino First Nation, particularly Chief Tom Nelson, and the planning staff, including Patrick Charlie, Leonard Williams and Aaron Wiliams. The input of Band Council consultants Robin Chatan and Dave Schmidt is also appreciated. -
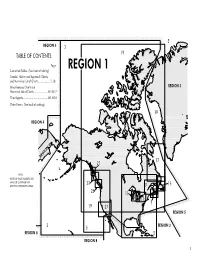
Index to NGA Charts, Region 1
1 2 REGION 1 COASTAL CHARTS Stock Number Title Scale =1: 11004 Mississippi River to Rio Grande 866,500 14003 Cape Race to Cape Henry 1,532,210 14018 The Grand Banks of Newfoundland and the Adjacent Coast 1,200,000 14024 Island of Newfoundland 720,240 15017 Hudson Strait (OMEGA) 1,000,000 15018 Belle Isle to Resolution Island (OMEGA) 1,000,000 15020 Hudson Strait to Greenland 1,501,493 15023 Queen Elizabeth Islands - Southern Part and Adjacent Waters 1,000,000 16220 St. Lawrence Island to Bering Strait 315,350 17003 Strait of Juan de Fuca to Dixon Entrance 1,250,000 18000 Point Conception to Isla Cedros 950,000 19008 Hawaiian Islands (OMEGA-BATHYMETRIC CHART) 1,030,000 38029 Baffin Bay (OMEGA) 917,000 38032 Godthabsfjord to Qeqertarsuaq including Cumberland Peninsula 841,000 38280 Kennedy Channel-Kane Basin to Hall Basin 300,000 38300 Smith Sound and Kane Basin 300,000 38320 Inglefield Bredning &Approaches 300,000 96028 Poluostrov Kamchatka to Aleutian Islands including Komandorskiye Ostrova 1,329,300 96036 Bering Strait (OMEGA) 928,770 3 4 REGION 1 COASTAL CHARTS EAST AND WEST COASTS-UNITED STATES Stock Number Title Scale =1: 11461 Straits of Florida-Southern Portion 300,000 13264 Approaches to Bay of Fundy 300,000 17005 Vancouver Island 525,000 17008 Queen Charlotte Sound to Dixon Entrance 525,000 17480 Queen Charlotte Sound 365,100 18766 San Diego to Islas De Todos Santos (LORAN-C) 180,000 5 6 NOVA SCOTIA AREA Stock Number Title Scale =1: Stock Number Title Scale =1: 14061 Grand Manan (Bay of Fundy) 60,000 14136 Sydney Harbour 20,000 14081 Medway Harbour to Lockeport Harbour including Liverpool 80,000 Plans: A. -

Marine Harvest Canada Monday Rock, Quatsino Sound
Aquaculture Stewardship Council Salmon Standard Final Assessment Report Non-confidential issue ASC Salmon Standard V1.0 June 2012 Marine Harvest Canada Monday Rock, Quatsino Sound Report Author: Paul Casburn Company: SAI Global Assurance Service Address: 3rd Floor Block 3 Quayside Business Park Mill Street Dundalk, Co. Louth Ireland Tel: +353 42 9320912 Fax: + 353 42 9386864 Correspondence: [email protected] W: www.saiglobal.com Report Code: ASC020 Report Release: 19th April 2016 Contents 1. Summary ....................................................................................................................... 3 2. CAB Contact Information ............................................................................................... 4 3. Background on the Applicant Farm ................................................................................ 4 4. Scope ............................................................................................................................ 5 5. Audit Plan ......................................................................................................................... 5 5.1 Auditors ....................................................................................................................... 5 5.2 Previous Audits............................................................................................................ 6 5.3 Audit Plan as Implemented .......................................................................................... 6 5.4 Staff Interviews -

Journal of Marine Research, Sears Foundation for Marine Research
The Journal of Marine Research is an online peer-reviewed journal that publishes original research on a broad array of topics in physical, biological, and chemical oceanography. In publication since 1937, it is one of the oldest journals in American marine science and occupies a unique niche within the ocean sciences, with a rich tradition and distinguished history as part of the Sears Foundation for Marine Research at Yale University. Past and current issues are available at journalofmarineresearch.org. Yale University provides access to these materials for educational and research purposes only. Copyright or other proprietary rights to content contained in this document may be held by individuals or entities other than, or in addition to, Yale University. You are solely responsible for determining the ownership of the copyright, and for obtaining permission for your intended use. Yale University makes no warranty that your distribution, reproduction, or other use of these materials will not infringe the rights of third parties. This work is licensed under the Creative Commons Attribution- NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA. Journal of Marine Research, Sears Foundation for Marine Research, Yale University PO Box 208118, New Haven, CT 06520-8118 USA (203) 432-3154 fax (203) 432-5872 [email protected] www.journalofmarineresearch.org SOME OCEANOGRAPIDC CHARACTERISTICS OF A POLLUTED INLET IN BRITISH COLUMBIA MICHAEL WALDICHUK Fi8heriea Research Board of Canada Biological S1.ation, Nanaimo, B.G. -

2018 ANNUAL REPORT 2 2018 Annual Report
2018 ANNUAL REPORT 2 2018 Annual Report About the SRD The Strathcona Regional District (SRD) is a partnership of five municipalities and four electoral areas, which covers approximately 22,000 square kilometers (8,517 square miles). The SRD serves and provides 44,671 residents (2016 census) with a diverse range of services including water and sewerage systems, fire protection, land use planning, parks, recreation and emergency response. The Strathcona Regional District was established on February 15, 2008, as a result of the provincial government’s restructure of the Comox Strathcona Regional District. The geography of the SRD ranges from forested hills, remote inlets, picturesque villages to vibrant urban landscapes. The borders extend from the Oyster River in the south to Gold River, Sayward, Tahsis, Zeballos and Kyuquot-Nootka in the north and west, and east to Cortes Island, Quadra Island and the Discovery Islands as well as a portion of the adjacent mainland north of Powell River. 128°0'0"W 127°0'0"W 126°0'0"W 125°0'0"W 124°0'0"W Stikine Fort Nelson-Liard Kitimat-Stikine er Peace River iv R o hk at m Skeena Buckley-Nechako o Queen Charlotte Fraser H Fort George 51°0'0"N Cariboo Central Coastal Columbia Q Shuswap u Thompson e Mount Waddington Nicola e Squamish North Lillooet Okanagan n Strathcona Powell Central East River Central Kootenay Kootenay Okanagan C Comox Hope Valley Sunshine Fraser Kootenay h Coast Valley Okanagan Boundary Greater Similkameen a Alberni Nanaimo Clayoquot Vancouver Island r Cowichan lo Valley t Capital 51°0'0"N -

Marine Distribution, Abundance, and Habitats of Marbled Murrelets in British Columbia
Chapter 29 Marine Distribution, Abundance, and Habitats of Marbled Murrelets in British Columbia Alan E. Burger1 Abstract: About 45,000-50,000 Marbled Murrelets (Brachyramphus birds) are extrapolations from relatively few data from 1972 marmoratus) breed in British Columbia, with some birds found in to 1982, mainly counts in high-density areas and transects most parts of the inshore coastline. A review of at-sea surveys at covering a small portion of coastline (Rodway and others 84 sites revealed major concentrations in summer in six areas. 1992). Between 1985 and 1993 many parts of the British Murrelets tend to leave these breeding areas in winter. Many Columbia coast were censused, usually by shoreline transects, murrelets overwinter in the Strait of Georgia and Puget Sound, but the wintering distribution is poorly known. Aggregations in sum- although methods and dates varied, making comparisons mer were associated with nearshore waters (<1 km from shore in and extrapolation difficult. Much of the 27,000 km of coastline exposed sites, but <3 km in sheltered waters), and tidal rapids and remains uncensused (fig. 1). narrows. Murrelets avoided deep fjord water. Several surveys showed Appendix 1 summarizes censuses for the core of the considerable daily and seasonal variation in densities, sometimes breeding season (1 May through 31 July). Murrelet densities linked with variable prey availability or local water temperatures. are given as birds per linear kilometer of transect. It was Anecdotal evidence suggests significant population declines in the necessary to convert density estimates from other units in Strait of Georgia, associated with heavy onshore logging in the several cases, and this was done in consultation with the early 1900s. -

Dinoflagellate Cysts in Recent Sediments from Fjords of Western Vancouver Island (British Columbia, Canada): Preliminary Results Pieter R
Dinoflagellate cysts in recent sediments from fjords of western Vancouver Island (British Columbia, Canada): preliminary results Pieter R. Gurdebekea, Vera Pospelovab, Kenneth N. Mertensa, Jasmin Chanab, Audrey Dallimorec & Stephen Louwyea a Research Unit Palaeontology, Departement of Geology and Soil Science, Ghent University. Building S8, Krijgslaan 281, 9000 Ghent, Belgium; bSchool of Earth and Ocean Sciences, University of Victoria, PO BOX 1700 STN CSC, Victoria, BC, V8W 2Y2, Canada; cGeological Survey of Canada-Pacific, PO Box 6000, Sidney, British Columbia V8L 4B2, Canada Surface sediment samples from coastal inlets of western Vancouver Island and shallow bays of the Broughton Archipelago were investigated for dinoflagellate cysts and other marine palynomorphs. Well preserved dinoflagellate cyst assemblages have been recovered and a total of 45 cyst types, representing 18 genera of 3 orders were identified. Cyst assemblages were dominated by Operculodinium centrocarpum, Spiniferistes spp. and Brigantedinium spp. Total dinoflagellate cyst concentrations vary two orders of magnitude from 7.489 to 918.584 cysts per gram of dry sediment, with the highest values observed in samples from Tofino Inlet. Cyst concentrations and assemblage diversity yielded higher values in Sydney Inlet in the Clayoquot Sound, Neroutos Inlet in the Quatsino Sound and Kashutl Inlet in the Kyuquot Sound, compared to the shallow bays of the Broughton Archipelago where the values were the lowest. Tofino Inlet had the highest abundance of Operculodinium centrocarpum, whereas Neroutos Inlet samples were characterized by high concentrations of Centropyxis cf. aculeatum, a testate amoeba. Cyst produced by heterotrophic dinoflagellates, mainly Brigantedinium spp. and indeterminate round brown cysts, dominated cyst assemblages in Sydney Inlet in the Clayoquot and in Amai Inlet in the Kyuquot Sound These sites were also characterized by the elevated values of sedimentary biogenic silica (BioSi).