Coronary Microvascular Dysfunction in HIV: a Review Leah Rethy, BA; Matthew J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Review Karen Bleich NDA 020351 Supplement 44 (CCTA) Visipaque (Iodixanol)
Clinical Review Karen Bleich NDA 020351 Supplement 44 (CCTA) Visipaque (iodixanol) CLINICAL REVIEW Application Type Supplemental New Drug Application Application Number(s) NDA 020351 s44 Priority or Standard Priority Submit Date(s) October 6th, 2016 Received Date(s) October 18th, 2016 PDUFA Goal Date April 5th, 2017 Division/Office Division of Medical Imaging Products/Office of Drug Evaluation IV Reviewer Name(s) Karen Bleich, MD Review Completion Date March 10th, 2017 Established Name Iodixanol (Proposed) Trade Name Visipaque Injection Applicant GE Healthcare Formulation(s) 320 mgI/mL Dosing Regimen 70-80 mL main bolus volume (does not include optional test bolus volume of 20 mL) at a flow rate of(b) (4) mL/s, followed by 20 mL saline flush Applicant Proposed For use in coronary computed tomography angiography (CCTA) to Indication(s)/Population(s) assist in the diagnostic evaluation of patients with suspected coronary artery disease. Recommendation on Approval Regulatory Action Recommended For use in coronary computed tomography angiography (CCTA) to Indication(s)/Population(s) assist in the diagnostic evaluation of patients with suspected (if applicable) coronary artery disease. CDER Clinical Review Template 2015 Edition 1 Reference ID: 4068412 Clinical Review Karen Bleich NDA 020351 Supplement 44 (CCTA) Visipaque (iodixanol) Table of Contents Glossary ........................................................................................................................................... 8 1 Executive Summary .............................................................................................................. -

Coronary Artery Calcium Quantification in Contrast-Enhanced Computed Tomography Angiography
Georgia State University ScholarWorks @ Georgia State University Computer Science Dissertations Department of Computer Science 12-18-2013 Coronary Artery Calcium Quantification in Contrast-enhanced Computed Tomography Angiography Abinashi Dhungel Georgia State University Follow this and additional works at: https://scholarworks.gsu.edu/cs_diss Recommended Citation Dhungel, Abinashi, "Coronary Artery Calcium Quantification in Contrast-enhanced Computed Tomography Angiography." Dissertation, Georgia State University, 2013. https://scholarworks.gsu.edu/cs_diss/80 This Dissertation is brought to you for free and open access by the Department of Computer Science at ScholarWorks @ Georgia State University. It has been accepted for inclusion in Computer Science Dissertations by an authorized administrator of ScholarWorks @ Georgia State University. For more information, please contact [email protected]. CORONARY ARTERY CALCIUM QUANTIFICATION IN CONTRAST-ENHANCED COMPUTED TOMOGRAPHY ANGIOGRAPHY by ABINASHI DHUNGEL Under the Direction of Dr. Michael Weeks ABSTRACT Coronary arteries are the blood vessels supplying oxygen-rich blood to the heart muscles. Coronary artery calcium (CAC), which is the total amount of calcium deposited in these arteries, indicates the presence or the future risk of coronary artery diseases. Quantification of CAC is done by using computed tomography (CT) scan which uses attenuation of x- ray by different tissues in the body to generate three-dimensional images. Calcium can be easily spotted in the CT images because of its higher opacity to x-ray compared to that of the surrounding tissue. However, the arteries cannot be identified easily in the CT images. Therefore, a second scan is done after injecting a patient with an x-ray opaque dye known as contrast material which makes different chambers of the heart and the coronary arteries visible in the CT scan. -

Influence of Cardiac Hemodynamic Parameters on Coronary Artery
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Eur Radiol (2006) 16: 1111–1116 DOI 10.1007/s00330-005-0110-4 CARDIAC Lars Husmann Influence of cardiac hemodynamic parameters Hatem Alkadhi Thomas Boehm on coronary artery opacification with 64-slice Sebastian Leschka Tiziano Schepis computed tomography Pascal Koepfli Lotus Desbiolles Borut Marincek Philipp A. Kaufmann Simon Wildermuth Abstract The purpose of this study artery. A significant negative correla- Received: 13 September 2005 Revised: 21 November 2005 was to evaluate the influence of tion was found in both arteries between Accepted: 29 November 2005 ejection fraction (EF), stroke volume SV and attenuation (RCA r=−0.26, Published online: 28 January 2006 (SV), heart rate, and cardiac output P<0.05; LMA r=−0.34, P<0.01) and # Springer-Verlag 2006 (CO) on coronary artery opacification between SV and CNR (RCA r=−0.26, L. Husmann . H. Alkadhi (*) . with 64-slice computed tomography P<0.05; LMA r=−0.26, P<0.05). T. Boehm . S. Leschka . (CT). Sixty patients underwent, retro- Similarly, a significant negative cor- L. Desbiolles . B. Marincek Department of Medical Radiology, spectively, electrocardiography-gated relation was found between the CO and Institute of Diagnostic Radiology, 64-slice CT coronary angiography. attenuation (RCA r=−0.42, P<0.05; University Hospital of Zurich, Left ventricular EF, SV, and CO LMA r=−0.56, P<0.001) and between Raemistrasse 100, were calculated with semi-automated the CO and CNR (RCA r=−0.39, 8091 Zurich, Switzerland software. -
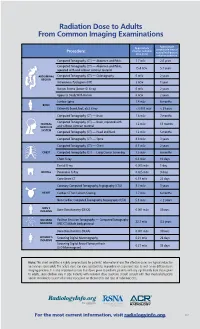
Radiation Dose to Adults from Common Imaging Examinations
Radiation Dose to Adults From Common Imaging Examinations Approximate Approximate comparable time of Procedure effective radiation natural background dose (mSv) radiation exposure Computed Tomography (CT) — Abdomen and Pelvis 7.7 mSv 2.6 years Computed Tomography (CT) — Abdomen and Pelvis, 15.4 mSv 5.1 years repeated with and without contrast material ABDOMINAL Computed Tomography (CT) — Colonography 6 mSv 2 years REGION Intravenous Pyelogram (IVP) 3 mSv 1 year Barium Enema (Lower GI X-ray) 6 mSv 2 years Upper GI Study With Barium 6 mSv 2 years Lumbar Spine 1.4 mSv 6 months BONE Extremity (hand, foot, etc.) X-ray < 0.001 mSv < 3 hours Computed Tomography (CT) — Brain 1.6 mSv 7 months Computed Tomography (CT) — Brain, repeated with CENTRAL 3.2 mSv 13 months NERVOUS and without contrast material SYSTEM Computed Tomography (CT) — Head and Neck 1.2 mSv 5 months Computed Tomography (CT) — Spine 8.8 mSv 3 years Computed Tomography (CT) — Chest 6.1 mSv 2 years CHEST Computed Tomography (CT) — Lung Cancer Screening 1.5 mSv 6 months Chest X-ray 0.1 mSv 10 days Dental X-ray 0.005 mSv 1 day DENTAL Panoramic X-Ray 0.025 mSv 3 days Cone Beam CT 0.18 mSv 22 days Coronary Computed Tomography Angiography (CTA) 8.7 mSv 3 years HEART Cardiac CT for Calcium Scoring 1.7 mSv 6 months Non-Cardiac Computed Tomography Angiography (CTA) 5.1 mSv < 2 years MEN’S IMAGING Bone Densitometry (DEXA) 0.001 mSv 3 hours NUCLEAR Positron Emission Tomography — Computed Tomography 22.7 mSv 3.3 years MEDICINE (PET/CT) Whole body protocol Bone Densitometry (DEXA) 0.001 mSv 3 hours WOMEN’S Screening Digital Mammography 0.21 mSv 26 days IMAGING Screening Digital Breast Tomosynthesis 0.27 mSv 33 days (3-D Mammogram) Note: This chart simplifies a highly complex topic for patients’ informational use. -

Coronary Artery Calcium Scoring – Position Statement
The Cardiac Society of Australia and New Zealand Coronary Artery Calcium Scoring – Position Statement Development of this position statement was coordinated by Christian Hamilton-Craig (co-chair), Gary Liew (co-chair), Jonathan Chan, Clara Chow, Michael Jelinek, Niels van Pelt and John Younger. No authors have any relevant Conflict of Interest to disclose. It was reviewed by the Quality Standards Committee and ratified at the CSANZ th Board meeting held on Friday, 26 May 2017. Coronary Artery Calcium Scoring (CAC) is a non-invasive quantitation of coronary artery calcification using computed tomography (CT). It is a marker of atherosclerotic plaque burden and an independent predictor of future myocardial infarction and mortality. CAC provides incremental risk information beyond traditional risk calculators (eg. Framingham Risk Score). Its use for risk stratification is confined to primary prevention of cardiovascular events, and can be considered as “individualized coronary risk scoring” for those not considered to be of high or low risk. Medical practitioners should carefully counsel patients prior to CAC. CAC should only be undertaken if an alteration in therapy including embarking on pharmacotherapy is being considered based on the test result. Patient groups to consider Coronary Calcium Scoring 1. CAC is of most value in intermediate risk patients (absolute 10-year cardiovascular risk of 10-20%) who are asymptomatic, do not have known coronary artery disease and aged 45 – 75 years, where it has the ability to reclassify patients into lower or higher risk groups. 2. It may also be considered for lower risk patients (absolute 10-year cardiovascular risk 6-10%) particularly in those where traditionally risk scores under estimate risk e.g. -

Physician Fee Schedule 2021 Note
Physician Fee Schedule 2021 Note: 2021 Codes in Red; Refer to CPT book for descriptions R" in PA column indicates Prior Auth is required Codes listed as '$0.00" pay 45% of billed amount not to exceed provider’s usual and customary charge for the service The Anesthesia Base Rate is $15.20. Each 15 minute increment=1 time unit. Please use lab fee schedule for covered codes not listed below in the 80000-89249 range. Codes listed on the lab fee schedule that begin with a P or Q are currently non-covered for physicians Proc Inpat. Rate Outpat. Rate Tech. Prof. Base Unit Code Procedure Description PA Ind (Facility) (NonFacility) Comp. Comp. Value Notes See Billing See Billing Manual Manual 00100 ANES FOR PROCEDURES ON SALIVARY GLANDS, INCLUDING BIOPSY Instructions Instructions 5 See Billing See Billing Manual Manual 00102 ANES FOR PROCEDURES INVOLVING PLASTIC REPAIR OF CLEFT LIP Instructions Instructions 6 See Billing See Billing Manual Manual 00103 ANES FOR RECONSTRUCTIVE PROCED OF EYELID Instructions Instructions 5 See Billing See Billing Manual Manual 00104 ANES FOR ELECTROCONVULSIVE THERAPY Instructions Instructions 4 See Billing See Billing ANES FOR PROC ON EXTERNAL, MIDDLE, AND INNER EAR ,INC Manual Manual 00120 BIOPSY Instructions Instructions 5 See Billing See Billing ANES FOR PROC ON EXTERNAL, MIDDLE, AND INNER Manual Manual 00124 EAR,OTOSCOPY Instructions Instructions 4 See Billing See Billing ANES FOR PROC ON EXTERNAL, MIDDLE, AND INNER EAR, Manual Manual 00126 TYMPANOTOMY Instructions Instructions 4 See Billing See Billing Manual -

Evicore Cardiac Imaging Guidelines V19.0
CLINICAL GUIDELINES Cardiac Imaging Policy Version 19.0 Effective May 22nd, 2017 eviCore healthcare Clinical Decision Support Tool Diagnostic Strategies: This tool addresses common symptoms and symptom complexes. Imaging requests for individuals with atypical symptoms or clinical presentations that are not specifically addressed will require physician review. Consultation with the referring physician, specialist and/or individual’s Primary Care Physician (PCP) may provide additional insight. CPT® (Current Procedural Terminology) is a registered trademark of the American Medical Association (AMA). CPT® five digit codes, nomenclature and other data are copyright 2016 American Medical Association. All Rights Reserved. No fee schedules, basic units, relative values or related listings are included in the CPT® book. AMA does not directly or indirectly practice medicine or dispense medical services. AMA assumes no liability for the data contained herein or not contained herein. © 2017 eviCore healthcare. All rights reserved. CARDIAC IMAGING GUIDELINES ABBREVIATIONS 3 GLOSSARY 4 ESTIMATE OF EFFECTIVE RADIATION DOSE CHART FOR SELECTED IMAGING STUDIES 4 CD-1~GENERAL GUIDELINES 5 CD-2~ECHOCARDIOGRAPHY (ECHO) 14 CD-3~NUCLEAR CARDIAC IMAGING 23 CD-3~NUCLEAR CARDIAC IMAGING 24 CD-4~ULTRAFAST CT, EBCT, OR MULTIDETECTOR CT FOR CORONARY CALCIUM SCORING (CCS) 29 CD-5~CARDIAC IMAGING BASED ON CORONARY CALCIUM SCORE 30 CD-6~CARDIAC MRI 31 CD-6~CARDIAC MRI 32 CD-7~CARDIAC PET 36 CD-8~CT HEART AND CCTA 38 CD-9~DIAGNOSTIC HEART CATHETERIZATION 44 CD-9~DIAGNOSTIC -

A Standardised CT Coronary Angiography Service Operated from a District General Hospital
Open access Health care delivery, economics and global health care Open Heart: first published as 10.1136/openhrt-2018-000817 on 11 July 2018. Downloaded from The HASTE Protocol: a standardised CT Coronary Angiography service operated from a District General Hospital Iain Thomas Parsons,1 Clare Bannister,1 John Badelek,2 Mark Ingram,2 Emma Wood,2 Alex Horton,2 Michael Hickman,1 Edward Leatham1 To cite: Parsons IT, Bannister C, ABSTRACT Key questions Badelek J, et al. The HASTE Introduction CT coronary angiography (CTCA) has Protocol: a standardised CT excellent sensitivity but lacks specificity when compared Coronary Angiography service What is already known about this subject? with invasive coronary angiography (ICA) particularly in operated from a District General CT coronary angiography is the non-invasive test of patients with a high coronary calcium burden. CTCA has ► Hospital. Open Heart choice for the investigation of stable coronary artery been shown in large trials to decrease the requirement for 2018;5:e000817. doi:10.1136/ disease. openhrt-2018-000817 diagnostic ICA and provide diagnostic clarity. We describe A considerable upscale in the provision of CT coro- the methodology used to provide a standardised CTCA ► nary angiography is required. service established in a District General Hospital, which Received 7 March 2018 may assist other hospitals aiming to develop a cardiac CT What does this study add? Revised 24 May 2018 service. A detailed description of the methodology for the Accepted 13 June 2018 ► Methods Scan request forms, authorisation and patient provision of a cardiac CT service from a District instruction were recorded. -

Appropriate Use Criteria for Cardiac Computed Tomography
ACCF/SCCT/ACR/AHA/ASE/ASNC/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society of Cardiovascular Computed Tomography, American College of Radiology, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society for Cardiovascular Magnetic Resonance, Allen J. Taylor, Manuel Cerqueira, John McB. Hodgson, Daniel Mark, James Min, Patrick O'Gara, and Geoffrey D. Rubin J. Am. Coll. Cardiol. published online Oct 25, 2010; doi:10.1016/j.jacc.2010.07.005 This information is current as of October 26, 2010 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://content.onlinejacc.org/cgi/content/full/j.jacc.2010.07.005v1 Downloaded from content.onlinejacc.org by on October 26, 2010 Journal of the American College of Cardiology Vol. 56, No. xx, 2010 © 2010 by the American College of Cardiology Foundation ISSN 0735-1097/$36.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2010.07.005 APPROPRIATE USE CRITERIA ACCF/SCCT/ACR/AHA/ASE/ASNC/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance Cardiac Allen J. Taylor, MD, FACC, FAHA, Chair* Computed *Official American College of Cardiology Foundation Representative; Tomography †Official American Society of Nuclear Cardiology Representative; Writing Group Manuel Cerqueira, MD, FACC, FASNC† ‡Official Society for Cardiovascular Angiography and Interventions John McB. -

1199SEIU Benefit Funds Cardiology & Radiology Code List
1199SEIU Benefit Funds Claims Management Procedure List: Cardiology & Radiology Included w/ Category CPT® Code CPT® Code Description Claims Management 3D rendering with interpretation and reporting of computed tomography, magnetic resonance imaging, ultrasound, 3D Imaging 76377 or other tomographic modality with image postprocessing under concurrent supervision; requiring image X postprocessing on an independent workstation 3D rendering with interpretation and reporting of computed tomography, magnetic resonance imaging, ultrasound, 3D Imaging 76376 or other tomographic modality with image postprocessing under concurrent supervision; not requiring image X postprocessing on an independent workstation Magnetic resonance imaging, breast, without and with contrast material(s), including computer-aided detection BMRI 77049 X (CAD real-time lesion detection, characterization and pharmacokinetic analysis), when performed; bilateral Magnetic resonance imaging, breast, without and with contrast material(s), including computer-aided detection BMRI 77048 X (CAD real-time lesion detection, characterization and pharmacokinetic analysis), when performed; unilateral BMRI 77047 Magnetic resonance imaging, breast, without contrast material; bilateral X BMRI 77046 Magnetic resonance imaging, breast, without contrast material; unilateral X Use of echocardiographic contrast agent during stress echocardiography (List separately in addition to code for Cardiac Services 93352 X primary procedure) Echocardiography, transthoracic, real-time with image documentation -

SCCT Guidelines for the Performance and Acquisition of Coronary
Journal of Cardiovascular Computed Tomography 10 (2016) 435e449 Contents lists available at ScienceDirect Journal of Cardiovascular Computed Tomography journal homepage: www.JournalofCardiovascularCT.com Guidelines SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the Society of Cardiovascular Computed Tomography Guidelines Committee Endorsed by the North American Society for Cardiovascular Imaging (NASCI) * Suhny Abbara a, , Philipp Blanke b, Christopher D. Maroules a, Michael Cheezum c, Andrew D. Choi d, B. Kelly Han e, Mohamed Marwan f, Chris Naoum g, Bjarne L. Norgaard h, Ronen Rubinshtein i, Paul Schoenhagen k, Todd Villines j, Jonathon Leipsic b a University of Texas Southwestern Medical Center, Dallas, TX, United States b Department of Radiology and Division of Cardiology, University of British Columbia, Vancouver, British Columbia, Canada c Cardiology Service Ft. Belvoir Community Hospital, Ft. Belvoir, VA, United States d Division of Cardiology and Department of Radiology, The George Washington University School of Medicine, Washington DC, United States e Minneapolis Heart Institute and Children's Heart Clinic, Minneapolis, MN, United States f Cardiology Department, University Hospital, Erlangen, Germany g Concord Hospital, The University of Sydney, Sydney, Australia h Department of Cardiology B, Aarhus University Hospital-Skejby, Aarhus N, Denmark i Lady Davis Carmel Medical Center & Rappaport School of Medicine- Technion- IIT, Haifa, Israel j Walter Reed National Military -

Coronary Artery Calcium Scoring and Cardiac Ct Angiography
MEDICAL POLICY CARDIAC COMPUTED TOMOGRAPHY (CT), CORONARY ARTERY CALCIUM SCORING AND CARDIAC CT ANGIOGRAPHY Policy Number: 2010T0488G Effective Date: May 28, 2010 Related Medical Policies: • Transthoracic Table of Contents Page Echocardiography • Single Photon COVERAGE RATIONALE........................................... 1 Emission Computed BENEFIT CONSIDERATIONS.................................... 4 Tomography CLINICAL EVIDENCE................................................. 4 Myocardial Perfusion U.S. FOOD AND DRUG ADMINISTRATION............... 19 Imaging (SPECT MPI) CENTERS FOR MEDICARE AND MEDICAID SERVICES (CMS)....................................................... 19 Related Coverage APPLICABLE CODES................................................. 20 Determination REFERENCES............................................................ 20 Guidelines: POLICY HISTORY/REVISION INFORMATION.......... 24 None Policy History Revision Information INSTRUCTIONS FOR USE This Medical policy provides assistance in interpreting UnitedHealthcare benefit plans. When deciding coverage, the enrollee specific document must be referenced. The terms of an enrollee's document (e.g., Certificate of Coverage (COC) or Summary Plan Description (SPD)) may differ greatly. In the event of a conflict, the enrollee's specific benefit document supersedes this medical policy. All reviewers must first identify enrollee eligibility, any federal or state regulatory requirements and the plan benefit coverage prior to use of this Medical Policy. Other Policies and