Physician Fee Schedule 2021 Note
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Is Optical Imaging?
What is Optical Imaging? Optical imaging uses light to interrogate cellular and molecular function in the living body, as well as in animal and plant tissue. The information is ultimately derived from tissue composition and biomolecular processes. Images are generated by using photons of light in the wavelength range from ultraviolet to near infrared. Contrast is derived through the use of: exogenous agents (i.e., dyes or probes) that provide a signal endogenous molecules with optical signatures (i.e., NADH, hemoglobin, collagens, etc.) reporter genes. Florescence Imaging Fluorescence protein imaging uses endogenous or exogenous molecules or materials that emit light when activated by an external light source such as a laser. An external light of appropriate wavelength is used to excite a target molecule, which then fluoresces by releasing longer-wavelength, lower-energy light. Fluorescence imaging provides the ability to localize and measure gene expression including normally expressed and aberrant genes, proteins and other pathophysiologic processes. Other potential uses include cell trafficking, tagging superficial structures, detecting lesions and for monitoring tumor growth and response to therapy. Bioluminescent Imaging (BLI) Bioluminescent imaging uses a natural light-emitting protein such as luciferase to trace the movement of certain cells or to identify the location of specific chemical reactions within the body. Bioluminescent imaging is being applied to both gene expression and therapeutic monitoring. Optical Imaging Technologies Near-infrared fluorescence imaging involves imaging fluorescence photons in the near-infrared range (typically 600– 900 nm). A fluorochrome is excited by a lower wavelength, light source and the emitted excitation is recorded as a slightly higher wavelength with a high sensitivity charge-coupled-device (CCD) camera. -

Clinical Update
Summer 2016 Clinical Update We are pleased to offer this archive of our award-winning newsletter Clinical Update. There are 75 issues in this document. Each issue has a feature article, summaries of articles in the nursing literature, and Web sites of interest. By downloading and using this archive, you agree that older medical articles may no longer describe appropriate practice. The issues are organized in date order from most recent to oldest. The following pages offer tips on how to navigate the issues and search the archive in Adobe Acrobat Reader. In 2006, we were honored to receive the Will Solimine Award of Excellence in Medical Writing from the American Medical Writers Association, New England Chapter. Issues that received the most positive response over the years include: • Nurses Removing Chest Tubes, a discussion of state boards of nursing’s approaches to this extended practice for registered nurses • Medical Adhesive Safety, a review of guidelines published by the Wound, Ostomy and Continence Nurses Society, complete with original tables identifying characteristics of each type of medical tape and how tape components contribute to medical adhesive- related skin injury (MARSI) • Autotransfusion for Jehovah’s Witness Patients, an explanation of the Biblical origins of the reasons for refusing blood transfusion and how continuous autotransfusion may offer an option that is acceptable to members of the faith • Air Transport for Patients with Chest Tubes and Pneumothorax and Chest Drainage and Hyperbaric Medicine, in which each issue provides a thorough analysis of how pressure changes with altitude and with increased atmospheric pressure affect chest drainage and untreated pneumothorax • Age Appropriate Competencies: Caring for Children that describes developmental stages and strategies to deal with a child’s fears at each stage Creative Commons License This work is licensed under a Creative Author: Patricia Carroll RN-BC, RRT, MS Commons Attribution-NonCommercial- ShareAlike 4.0 International License. -

Core Neurosurgery
BAYLOR SCOTT & WHITE TEXAS SPINE & JOINT HOSPITAL NEUROLOGICAL SURGERY CLINICAL PRIVILEGES NAME: ________________________________ Initial appointment Reappointment All new applicants must meet the following requirements as approved by the governing body. To be eligible to apply for core privileges in neurological surgery, the initial applicant must meet the following criteria: Successful completion of ACGME or American Osteopathic Association accredited residency in neurological surgery. Required previous experience: Applicants for initial appointment must be able to demonstrate the performance of at least 50 neurological surgical procedures, reflective of the scope of privileges requested, during the last 12 months or demonstrate successful completion of residency or fellowship within the past 12 months. Reappointment requirements: To be eligible to renew core privileges in Neurological Surgery, the applicant must meet the following maintenance of privilege criteria: Current demonstrated competence and an adequate volume of neurological surgery procedures with acceptable results, reflective of the scope of privileges requested, for the past 24 months based on results of ongoing professional practice evaluation and outcomes. Evidence of current ability to perform privileges requested is required of all applicants for renewal of privileges NEUROLOGICAL SURGERY CORE PRIVILEGES Requested: Admit, evaluate, diagnose, consult and provide nonoperative and pre-, intran, and postoperative care to patients of all ages presenting with injuries -

National Correct Coding Initiative Policy Manual for Medicare Services Revision Date: 1/1/2019
National Correct Coding Initiative Policy Manual for Medicare Services Revision Date: 1/1/2019 Current Procedural Terminology (CPT) codes, descriptions and other data only are copyright 2018 American Medical Association. All rights reserved. CPT® is a registered trademark of the American Medical Association. Applicable FARS\DFARS Restrictions Apply to Government Use. Fee schedules, relative value units, conversion factors, prospective payment systems, and/or related components are not assigned by the AMA, are not part of CPT, and the AMA is not recommending their use. The AMA does not directly or indirectly practice medicine or dispense medical services. The AMA assumes no liability for the data contained or not contained herein. TABLE OF CONTENTS FOR NATIONAL CORRECT CODING INITIATIVE POLICY MANUAL FOR MEDICARE SERVICES Introduction Introduction Intro-3 Purpose Intro-3 Policy Manual Background Intro-6 Edit Development and Review Process Intro-7 Sources of Information about NCCI and MUE Intro-9 Correspondence to CMS about NCCI and its Contents Intro-10 Chapter I - General Correct Coding Policies List of Acronyms I-3 A. Introduction I-5 B. Coding Based on Standards of Medical/Surgical I-9 Practice C. Medical/Surgical Package I-12 D. Evaluation and Management (E&M) Services I-17 E. Modifiers and Modifier Indicators I-19 F. Standard Preparation/Monitoring Services for I-27 Anesthesia G. Anesthesia Service Included in the Surgical Procedure I-27 H. HCPCS/CPT Procedure Code Definition I-28 I. CPT Manual and CMS Coding Manual Instructions I-29 J. CPT “Separate Procedure” Definition I-30 K. Family of Codes I-30 L. -

Neurosurgery
KALEIDA HEALTH Name ____________________________________ Date _____________ DELINEATION OF PRIVILEGES - NEUROSURGERY All members of the Department of Neurosurgery at Kaleida Health must have the following credentials: 1. Successful completion of an ACGME accredited Residency, Royal College of Physicians and Surgeons of Canada, or an ACGME equivalent Neurosurgery Residency Program. 2. Members of the clinical service of Neurosurgery must, within five (5) years of appointment to staff, achieve board certification in Neurosurgery. *Maintenance of board certification is mandatory for all providers who have achieved this status* Level 1 (core) privileges are those able to be performed after successful completion of an accredited Neurosurgery Residency program. The removal or restriction of these privileges would require further investigation as to the individual’s overall ability to practice, but there is no need to delineate these privileges individually. PLEASE NOTE: Please check the box for each privilege requested. Do not use an arrow or line to make selections. We will return applications that ignore this directive. LEVEL I (CORE) PRIVILEGES Basic Procedures including: Admission and Follow-Up Repair cranial or dural defect or lesion History and Physical for diagnosis and treatment plan* Seizure Chest tube placement Sterotactic framed localization of lesion Debride wound Sterotactic frameless localization Endotracheal intubation Transsphenoidal surgery of pituitary lesion Excision of foreign body Trauma Insertion of percutaneous arterial -

Colorectal Cancer Screening
CLINICAL MEDICAL POLICY Policy Name: Colorectal Cancer Screening Policy Number: MP-059-MD-PA Responsible Department(s): Medical Management Provider Notice Date: 03/19/2021 Issue Date: 03/19/2021 Effective Date: 04/19/2021 Next Annual Review: 02/2022 Revision Date: 02/17/2021 Products: Gateway Health℠ Medicaid Application: All participating hospitals and providers Page Number(s): 1 of 10 DISCLAIMER Gateway Health℠ (Gateway) medical policy is intended to serve only as a general reference resource regarding coverage for the services described. This policy does not constitute medical advice and is not intended to govern or otherwise influence medical decisions. POLICY STATEMENT Gateway Health℠ may provide coverage under the medical-surgical benefits of the Company’s Medicaid products for medically necessary colorectal cancer screening procedures. This policy is designed to address medical necessity guidelines that are appropriate for the majority of individuals with a particular disease, illness or condition. Each person’s unique clinical circumstances warrant individual consideration, based upon review of applicable medical records. (Current applicable Pennsylvania HealthChoices Agreement Section V. Program Requirements, B. Prior Authorization of Services, 1. General Prior Authorization Requirements.) Policy No. MP-059-MD-PA Page 1 of 10 DEFINITIONS Average-Risk Population – Patient population defined as having no personal history of adenomatous polyps, colorectal cancer, or inflammatory bowel disease (Crohn’s disease and Ulcerative Colitis); no family history of colorectal cancer or adenomatous polyps, familial adenomatous polyposis, or hereditary nonpolyposis colorectal cancer. High-Risk Population – Patient population defined as having a first-degree relative (sibling, parent, or child) who has had colorectal cancer or adenomatous polyps; OR family history of familial adenomatous polyposis; OR family history of hereditary non-polyposis colorectal cancer; OR family history of MYH- associated polyposis in siblings; OR diagnosis of Cowden syndrome. -

Nasal Airflow Measured by Rhinomanometry Correlates with Feno in Children with Asthma
RESEARCH ARTICLE Nasal Airflow Measured by Rhinomanometry Correlates with FeNO in Children with Asthma I-Chen Chen1☯, Yu-Tsai Lin2☯, Jong-Hau Hsu1,3, Yi-Ching Liu1, Jiunn-Ren Wu1,3, Zen- Kong Dai1,3* 1 Department of Pediatrics, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan, 2 Department of Otolaryngology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan, 3 Department of Pediatrics, School of Medicine, College of Medicine, a11111 Kaohsiung Medical University, Kaohsiung, Taiwan ☯ These authors contributed equally to this work. * [email protected] Abstract OPEN ACCESS Citation: Chen I-C, Lin Y-T, Hsu J-H, Liu Y-C, Wu Background J-R, Dai Z-K (2016) Nasal Airflow Measured by Rhinitis and asthma share similar immunopathological features. Rhinomanometry is an Rhinomanometry Correlates with FeNO in Children with Asthma. PLoS ONE 11(10): e0165440. important test used to assess nasal function and spirometry is an important tool used in doi:10.1371/journal.pone.0165440 asthmatic children. The degree to which the readouts of these tests are correlated has yet Editor: Stelios Loukides, National and Kapodistrian to be established. We sought to clarify the relationship between rhinomanometry measure- University of Athens, GREECE ments, fractional exhaled nitric oxide (FeNO), and spirometric measurements in asthmatic Received: September 3, 2016 children. Accepted: October 11, 2016 Methods Published: October 28, 2016 Patients' inclusion criteria: age between 5 and 18 years, history of asthma with nasal symp- Copyright: © 2016 Chen et al. This is an open toms, and no anatomical deformities. All participants underwent rhinomanometric evalua- access article distributed under the terms of the Creative Commons Attribution License, which tions and pulmonary function and FeNO tests. -

Diagnostic Nasal/Sinus Endoscopy, Functional Endoscopic Sinus Surgery (FESS) and Turbinectomy
Medical Coverage Policy Effective Date ............................................. 7/10/2021 Next Review Date ....................................... 3/15/2022 Coverage Policy Number .................................. 0554 Diagnostic Nasal/Sinus Endoscopy, Functional Endoscopic Sinus Surgery (FESS) and Turbinectomy Table of Contents Related Coverage Resources Overview .............................................................. 1 Balloon Sinus Ostial Dilation for Chronic Sinusitis and Coverage Policy ................................................... 2 Eustachian Tube Dilation General Background ............................................ 3 Drug-Eluting Devices for Use Following Endoscopic Medicare Coverage Determinations .................. 10 Sinus Surgery Coding/Billing Information .................................. 10 Rhinoplasty, Vestibular Stenosis Repair and Septoplasty References ........................................................ 28 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence -
FAQ Document
The Society of Thoracic Surgeons Frequently Asked Questions: General Thoracic Database Version 2.07 December 2008 How to use the “interactive” FAQ Document: 1. To review all clinical questions in an individual section, click on the section title below. Section A: seq# 10 - 190 Section C: seq# 285 - 650 Section E: seq# 770 - 1210 Section B: seq# 200 - 260 Section D: seq# 660 - 750 Section F: seq# 1220 - 1340 2. To review an individual Seq# clinical question, click on the Seq# title below. Participation in both General Thoracic and Adult Cardiac Databases GENERAL STATEMENT #2 Seq# 200: Zubrod Score Seq# 775: Postop Events Seq# 300: WtLoss3Kg Seq# 860: Pneumonia Seq# 310: Category of disease Seq# 930: Other Pulmonary Event Seq# 390: PreOp Chemotherapy Seq# 940: Atrial Arrhythmia Seq# 400: PreOp Thoracic RT Seq# 1020: Anastomotic leak Seq# 430: Other Cormorbidity Seq# 1190: Empyema Seq# 540: Clinical Stage Not Applicable (2.06) Seq# 1200: Other event req. Rx Seq# 725: Reoperation Seq# 1250: 30 Day Status Seq# 740: Procedure Seq# 1280: Chest Tube Out Date Seq# 750: Primary Procedure Seq# 751: Thoracoscopy Approach NEW Date SeqNo FieldName Definition 3/06 Participation in both General We participate in both Adult Cardiac and Yes, enter into both. In the Adult Cardiac DB, the Thoracic and Adult Cardiac General Thoracic Database. Our question primary procedure would be Seq# 1310 pertains to whether the patient should end up OpOCard=Yes; Seq# 2510 ONCAoAn=Yes; Databases in both databases. Scenario--We had a Seq# 2530 ONCArch=Yes; Seq# 2540 patient who had an aortic aneurysm repair ONCDesc=Yes; Seq# 3220 Readm30=Yes; (arch/descending thoracic) and was entered Seq# ReadmRsn=either Pneumonia or other into the Adult Cardiac Database. -

Clinical Review Karen Bleich NDA 020351 Supplement 44 (CCTA) Visipaque (Iodixanol)
Clinical Review Karen Bleich NDA 020351 Supplement 44 (CCTA) Visipaque (iodixanol) CLINICAL REVIEW Application Type Supplemental New Drug Application Application Number(s) NDA 020351 s44 Priority or Standard Priority Submit Date(s) October 6th, 2016 Received Date(s) October 18th, 2016 PDUFA Goal Date April 5th, 2017 Division/Office Division of Medical Imaging Products/Office of Drug Evaluation IV Reviewer Name(s) Karen Bleich, MD Review Completion Date March 10th, 2017 Established Name Iodixanol (Proposed) Trade Name Visipaque Injection Applicant GE Healthcare Formulation(s) 320 mgI/mL Dosing Regimen 70-80 mL main bolus volume (does not include optional test bolus volume of 20 mL) at a flow rate of(b) (4) mL/s, followed by 20 mL saline flush Applicant Proposed For use in coronary computed tomography angiography (CCTA) to Indication(s)/Population(s) assist in the diagnostic evaluation of patients with suspected coronary artery disease. Recommendation on Approval Regulatory Action Recommended For use in coronary computed tomography angiography (CCTA) to Indication(s)/Population(s) assist in the diagnostic evaluation of patients with suspected (if applicable) coronary artery disease. CDER Clinical Review Template 2015 Edition 1 Reference ID: 4068412 Clinical Review Karen Bleich NDA 020351 Supplement 44 (CCTA) Visipaque (iodixanol) Table of Contents Glossary ........................................................................................................................................... 8 1 Executive Summary .............................................................................................................. -
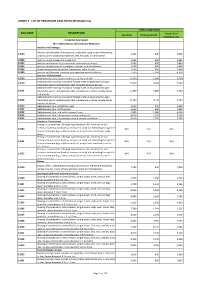
Annex 2. List of Procedure Case Rates (Revision 2.0)
ANNEX 2. LIST OF PROCEDURE CASE RATES (REVISION 2.0) FIRST CASE RATE RVS CODE DESCRIPTION Health Care Case Rate Professional Fee Institution Fee Integumentary System Skin, Subcutaneous and Accessory Structures Incision and Drainage Incision and drainage of abscess (e.g., carbuncle, suppurative hidradenitis, 10060 3,640 840 2,800 cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia) 10080 Incision and drainage of pilonidal cyst 3,640 840 2,800 10120 Incision and removal of foreign body, subcutaneous tissues 3,640 840 2,800 10140 Incision and drainage of hematoma, seroma, or fluid collection 3,640 840 2,800 10160 Puncture aspiration of abscess, hematoma, bulla, or cyst 3,640 840 2,800 10180 Incision and drainage, complex, postoperative wound infection 5,560 1,260 4,300 Excision - Debridement 11000 Debridement of extensive eczematous or infected skin 10,540 5,040 5,500 Debridement including removal of foreign material associated w/ open 11010 10,540 5,040 5,500 fracture(s) and/or dislocation(s); skin and subcutaneous tissues Debridement including removal of foreign material associated w/ open 11011 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 11,980 5,880 6,100 and muscle Debridement including removal of foreign material associated w/ open 11012 fracture(s) and/or dislocation(s); skin, subcutaneous tissue, muscle fascia, 12,120 6,720 5,400 muscle, and bone 11040 Debridement; skin, partial thickness 3,640 840 2,800 11041 Debridement; skin, full thickness 3,640 840 2,800 11042 Debridement; skin, and -

ICD~10~PCS Complete Code Set Procedural Coding System Sample
ICD~10~PCS Complete Code Set Procedural Coding System Sample Table.of.Contents Preface....................................................................................00 Mouth and Throat ............................................................................. 00 Introducton...........................................................................00 Gastrointestinal System .................................................................. 00 Hepatobiliary System and Pancreas ........................................... 00 What is ICD-10-PCS? ........................................................................ 00 Endocrine System ............................................................................. 00 ICD-10-PCS Code Structure ........................................................... 00 Skin and Breast .................................................................................. 00 ICD-10-PCS Design ........................................................................... 00 Subcutaneous Tissue and Fascia ................................................. 00 ICD-10-PCS Additional Characteristics ...................................... 00 Muscles ................................................................................................. 00 ICD-10-PCS Applications ................................................................ 00 Tendons ................................................................................................ 00 Understandng.Root.Operatons..........................................00