Revision 1 a Single-Crystal Neutron Diffraction Study of Wardite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -
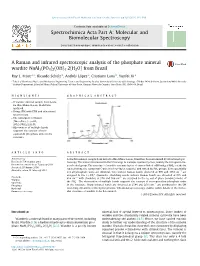
A Raman and Infrared Spectroscopic Analysis of the Phosphate Mineral
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 126 (2014) 164–169 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A Raman and infrared spectroscopic analysis of the phosphate mineral wardite NaAl3(PO4)2(OH)4Á2(H2O) from Brazil ⇑ Ray L. Frost a, , Ricardo Scholz b, Andrés López a, Cristiano Lana b, Yunfei Xi a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35400-00, Brazil highlights graphical abstract A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil was analysed. Using SEM with EDX and vibrational spectroscopy. The calculated formula is (Na0.97Ca0.03)R1.00Al3 (PO4)2(OH)4Á2(H2O). Observation of multiple bands supports the concept of non- equivalent phosphate units in the structure. article info abstract Article history: A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil has been examined by vibrational spec- Received 17 November 2013 troscopy. The mineral is unusual in that it belongs to a unique symmetry class, namely the tetragonal-tra- Received in revised form 7 January 2014 pezohedral group. The structure of wardite contains layers of corner-linked –OH bridged MO6 octahedra Accepted 2 February 2014 stacked along the tetragonal C-axis in a four-layer sequence and linked by PO groups. Consequentially Available online 15 February 2014 4 not all phosphate units are identical. -

38Th RMS Program Notes
E.fu\wsoil 'og PROGRAM Thursday Evening, April 14, 2011 PM 4:00-6:00 Cocktails and Snacks – Hospitality Suite 400 (4th Floor) 6:00-7:45 Dinner – Baxter’s 8:00-9:15 THE GUALTERONI COLLECTION: A TIME CAPSULE FROM A CENTURY AGO – Dr. Renato Pagano In 1950, the honorary curator of the Museum of Natural History in Genoa first introduced Dr. Renato Pagano to mineral collecting as a Boy Scout. He has never looked back. He holds a doctorate in electrical engineering and had a distinguished career as an Italian industrialist. His passion for minerals has produced a collection of more than 13,000 specimens, with both systematic and aesthetic subcollections. His wife Adriana shares his passion for minerals and is his partner in collecting and curating. An excellent profile of Renato, Adriana, and their many collections appeared earlier this year in Mineralogical Record (42:41-52). Tonight Dr. Pagano will talk about an historic mineral collection assembled between 1861 and 1908 and recently acquired intact by the Museum of Natural History of Milan. We most warmly welcome Dr. Renato Pagano back to the speakers’ podium. 9:15 Cocktails and snacks in the Hospitality Suite on the 4th floor will be available throughout the rest of the evening. Dealers’ rooms will be open at this time. All of the dealers are located on the 4th floor. Friday Morning, April 15, 2011 AM 9:00 Announcements 9:15-10:15 CRACKING THE CODE OF PHLOGOPITE DEPOSITS IN QUÉBEC (PARKER MINE), MADAGASCAR (AMPANDANDRAVA) AND RUSSIA (KOVDOR) – Dr. Robert F. Martin Robert François Martin is an emeritus professor of geology at McGill University in Montreal. -

A Vibrational Spectroscopic Study of the Phosphate Mineral Vantasselite
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 147 (2015) 185–192 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A vibrational spectroscopic study of the phosphate mineral vantasselite Al4(PO4)3(OH)3Á9H2O ⇑ Ray L. Frost a, , Ricardo Scholz b, Fernanda Maria Belotti c, Andrés López a, Frederick L. Theiss a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35,400-00, Brazil c Federal University of Itajubá, Campus Itabira, Itabira, MG 35,903-087, Brazil highlights graphical abstract We have studied the phosphate mineral vantasselite Al2(PO4)(OH)3Á3H2O. Using a combination of SEM with EDX and Raman and infrared spectroscopy. Chemical analysis shows a mineral containing Al, Fe and P. A comparison is made with other aluminum containing phosphate minerals. Vibrational spectroscopy offers a mechanism for the study of the molecular structure of vantasselite. article info abstract Article history: We have studied the phosphate mineral vantasselite Al4(PO4)3(OH)3Á9H2O using a combination of SEM Received 28 October 2014 with EDX and Raman and infrared spectroscopy. Qualitative chemical analysis shows Al, Fe and P. Received in revised form 15 January 2015 À1 3À Raman bands at 1013 and 1027 cm are assigned to the PO4 m1 symmetric stretching mode. The obser- Accepted 23 March 2015 vation of two bands suggests the non-equivalence of the phosphate units in the vantasselite structure. -

Fluorowardite, Naal3(PO4)2(OH)2F2·2H2O, the Fluorine Analog of Wardite from the Silver Coin Mine, Valmy, Nevada
American Mineralogist, Volume 99, pages 804–810, 2014 Fluorowardite, NaAl3(PO4)2(OH)2F22 Anthony R. KAmpf1,*, pAul m. AdAms2, RobeRt m. housley3 And GeoRGe R. RossmAn3 1Mineral Sciences Department, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, U.S.A. 2126 South Helberta Avenue, #2, Redondo Beach, California 90277, U.S.A. 3Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, U.S.A. AbstRAct Fluorowardite (IMA2012-016), NaAl3(PO4)2(OH)2F2·2H2O, the F analog of wardite, is a new mineral from the Silver Coin mine, Valmy, Iron Point district, Humboldt County, Nevada, U.S.A., where it oc- curs as a low-temperature secondary mineral in complex phosphate assemblages rich in Al, Na, and F. Fluorowardite forms colorless to white or cream-colored, tetragonal-pyramidal crystals up to 0.1 mm in diameter. The streak is white. Crystals are transparent to translucent, with vitreous to pearly luster. The Mohs hardness is about 5, the tenacity is brittle, the fracture is irregular, and crystals exhibit one perfect cleavage on {001}. The calculated density is 2.760 g/cm3 analyses (average of 8) provided: Na2O 6.27, CaO 1.74, MgO 0.42, Al2O3 35.21, Fe2O3 0.72, P2O5 32.49, As2O52O 13.35 (structure), total 94.74 wt%. The presence of H2O and OH and the absence of CO3 3+ anions) is: (Na0.87Ca0.13Mg0.04)(Al2.96Fe0.04)(P1.96As0.03)O8.12(OH)2.35F1.53·2H2O. Fluorowardite 3 is tetragonal, P41212, acV , and Z lines in the X-ray powder diffraction pattern are [dobsI)(hkl)]: 4.766(100)(004,103); 3.099(75) (211,203); 3.008(62)(115,212); 2.834(28)(204,213); 2.597(56)(205); 1.7628(32)(400,401); 1.6592(29) R1FoF) 62O) octahedra, PO4 tetrahedra, and NaO6(H2O)26 octahedra link by corner-sharing to form a square array. -

Roscherite-Group Minerals from Brazil
■ ■ Roscherite-Group Minerals yÜÉÅ UÜté|Ä Daniel Atencio* and José M.V. Coutinho Instituto de Geociências, Universidade de São Paulo, Rua do Lago, 562, 05508-080 – São Paulo, SP, Brazil. *e-mail: [email protected] Luiz A.D. Menezes Filho Rua Esmeralda, 534 – Prado, 30410-080 - Belo Horizonte, MG, Brazil. INTRODUCTION The three currently recognized members of the roscherite group are roscherite (Mn2+ analog), zanazziite (Mg analog), and greifensteinite (Fe2+ analog). These three species are monoclinic but triclinic variations have also been described (Fanfani et al. 1977, Leavens et al. 1990). Previously reported Brazilian occurrences of roscherite-group minerals include the Sapucaia mine, Lavra do Ênio, Alto Serra Branca, the Córrego Frio pegmatite, the Lavra da Ilha pegmatite, and the Pirineus mine. We report here the following three additional occurrences: the Pomarolli farm, Lavra do Telírio, and São Geraldo do Baixio. We also note the existence of a fourth member of the group, an as-yet undescribed monoclinic Fe3+-dominant species with higher refractive indices. The formulas are as follows, including a possible formula for the new species: Roscherite Ca2Mn5Be4(PO4)6(OH)4 • 6H2O Zanazziite Ca2Mg5Be4(PO4)6(OH)4 • 6H2O 2+ Greifensteinite Ca2Fe 5Be4(PO4)6(OH)4 • 6H2O 3+ 3+ Fe -dominant Ca2Fe 3.33Be4(PO4)6(OH)4 • 6H2O ■ 1 ■ Axis, Volume 1, Number 6 (2005) www.MineralogicalRecord.com ■ ■ THE OCCURRENCES Alto Serra Branca, Pedra Lavrada, Paraíba Unanalyzed “roscherite” was reported by Farias and Silva (1986) from the Alto Serra Branca granite pegmatite, 11 km southwest of Pedra Lavrada, Paraíba state, associated with several other phosphates including triphylite, lithiophilite, amblygonite, tavorite, zwieselite, rockbridgeite, huréaulite, phosphosiderite, variscite, cyrilovite and mitridatite. -
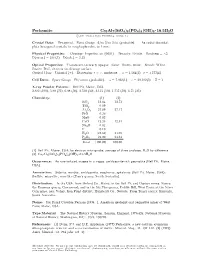
Perhamite Ca3al7(Sio4)3(PO4)4(OH)3 ² 16:5H2O C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Hexagonal
Perhamite Ca3Al7(SiO4)3(PO4)4(OH)3 ² 16:5H2O c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Hexagonal. Point Group: 6=m 2=m 2=m (probable). As radial discoidal, platy hexagonal crystals, in rough spherules, to 1 mm. Physical Properties: Cleavage: Imperfect on 0001 . Tenacity: Brittle. Hardness = 5 f g » D(meas.) = 2.64(1) D(calc.) = 2.53 Optical Properties: Translucent to nearly opaque. Color: Brown, white. Streak: White. Luster: Dull, vitreous on cleavage surface. Optical Class: Uniaxial (+). Dispersion: r > v; moderate. ! = 1.564(2) ² = 1.577(4) Cell Data: Space Group: P 6=mmm (probable). a = 7.022(1) c = 20.182(5) Z = 1 X-ray Powder Pattern: Bell Pit, Maine, USA. 2.882 (100), 5.80 (71), 6.08 (50), 3.510 (50), 3.115 (50), 1.757 (50), 6.71 (35) Chemistry: (1) (2) SiO2 13.64 13.72 TiO2 0.09 Al2O3 27.09 27.17 FeO 0.26 MgO 0.02 CaO 12.26 12.81 Na2O 0.02 F 0.10 H2O [24.62] 24.69 P2O5 21.90 21.61 Total [100.00] 100.00 (1) Bell Pit, Maine, USA; by electron microprobe, average of three analyses, H2O by di®erence. (2) Ca3Al7(SiO4)3(PO4)4(OH)3 ² 16:5H2O Occurrence: As rare isolated masses in a vuggy, amblygonite-rich pegmatite (Bell Pit, Maine, USA). Association: Siderite, wardite, amblygonite, eosphorite, sphalerite (Bell Pit, Maine, USA); °uellite, minyulite, wavellite (Tom's quarry, South Australia). Distribution: In the USA, from Oxford Co., Maine, in the Bell Pit and Dunton mines, Newry; the Emmons quarry, Greenwood; and in the Ski Pike quarry, Cobble Hill, West Paris; at the Silver Coin mine, near Valmy, Iron Point district, Humboldt Co., Nevada. -

Fluorowardite Naal3(PO4)2(OH)2F2·2H2O
Fluorowardite NaAl3(PO4)2(OH)2F2·2H2O Crystal Data: Tetragonal. Point Group: 422. As tetragonal bipyramidal crystals, to 0.1 mm, displaying {001} (prominent and lustrous), {011} and/or {012} (prominent, irregular, and striated parallel to [100]), and {100} (common, irregular, and striated parallel to [100]). Physical Properties: Cleavage: Perfect on {001}. Fracture: Irregular. Tenacity: Brittle. Hardness = 5 D(meas.) = n.d. D(calc.) = 2.706 Optical Properties: Transparent to translucent. Color: Colorless to white or cream-colored. Streak: White. Luster: Vitreous to pearly. Optical Class: Uniaxial (+). ω = 1.576(2) ε = 1.584(2) Cell Data: Space Group: P41212. a = 7.077(2) c = 19.227(3) Z = 4 X-ray Powder Pattern: Silver Coin mine, Valmy, Humboldt County, Nevada, USA. 4.766 (100), 3.099 (75), 3.008 (62), 2.597 (56), 1.5228 (49), 1.7628 (32), 1.6592 (29) Chemistry: (1) (2) Na2O 6.27 7.71 CaO 1.74 MgO 0.42 Al2O3 35.21 38.05 Fe2O3 0.72 P2O5 32.49 35.32 As2O5 0.64 F 6.76 9.45 -O = F2 2.85 3.98 H2O [13.35] 13.45 total 94.74 100.00 (1) Silver Coin mine, Valmy, Humboldt County, Nevada, USA; average of 8 electron microprobe analyses supplemented by FTIR and Raman spectroscopy, H2O calculated from the structure; 3+ corresponds to (Na0.87Ca0.13Mg0.04)Σ=1.04(Al2.96Fe 0.04)Σ=3.00(P1.96As0.03)Σ=1.99O8.12(OH)2.35F1.53·2H2O. (2) NaAl3(PO4)2(OH)2F2·2H2O. Occurrence: A secondary phase, in a F-rich secondary phosphate assemblage. -

STRONG and WEAK INTERLAYER INTERACTIONS of TWO-DIMENSIONAL MATERIALS and THEIR ASSEMBLIES Tyler William Farnsworth a Dissertati
STRONG AND WEAK INTERLAYER INTERACTIONS OF TWO-DIMENSIONAL MATERIALS AND THEIR ASSEMBLIES Tyler William Farnsworth A dissertation submitted to the faculty at the University of North Carolina at Chapel Hill in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Chemistry. Chapel Hill 2018 Approved by: Scott C. Warren James F. Cahoon Wei You Joanna M. Atkin Matthew K. Brennaman © 2018 Tyler William Farnsworth ALL RIGHTS RESERVED ii ABSTRACT Tyler William Farnsworth: Strong and weak interlayer interactions of two-dimensional materials and their assemblies (Under the direction of Scott C. Warren) The ability to control the properties of a macroscopic material through systematic modification of its component parts is a central theme in materials science. This concept is exemplified by the assembly of quantum dots into 3D solids, but the application of similar design principles to other quantum-confined systems, namely 2D materials, remains largely unexplored. Here I demonstrate that solution-processed 2D semiconductors retain their quantum-confined properties even when assembled into electrically conductive, thick films. Structural investigations show how this behavior is caused by turbostratic disorder and interlayer adsorbates, which weaken interlayer interactions and allow access to a quantum- confined but electronically coupled state. I generalize these findings to use a variety of 2D building blocks to create electrically conductive 3D solids with virtually any band gap. I next introduce a strategy for discovering new 2D materials. Previous efforts to identify novel 2D materials were limited to van der Waals layered materials, but I demonstrate that layered crystals with strong interlayer interactions can be exfoliated into few-layer or monolayer materials. -

Bobdownsite, a New Mineral Species from Big Fish River, Yukon, Canada, and Its Structural Relationship with Whitlockite-Type Compounds
1065 The Canadian Mineralogist Vol. 49, pp. 1065-1078 (2011) DOI : 10.3749/canmin.49.4.1065 BOBDOWNSITE, A NEW MINERAL SPECIES FROM BIG FISH RIVER, YUKON, CANADA, AND ITS STRUCTURAL RELATIONSHIP WITH WHITLOCKITE-TYPE COMPOUNDS KIMBERLY T. TAIT§ Department of Natural History, Royal Ontario Museum, 100 Queen’s Park, Toronto, Ontario M5S 2C6, Canada MADISON C. BARKLEY, RICHARD M. THOMPSON, MARCUS J. ORIGLIERI, StaNLEY H. EVANS, CHARLES T. PREWITT AND HEXIONG YANG Department of Geosciences, University of Arizona, 1040 E. 4th Street, Tucson, Arizona 85721–0077, U.S.A. ABSTRACT A new mineral species, bobdownsite, the F-dominant analogue of whitlockite, ideally Ca9Mg(PO4)6(PO3F), has been found in Lower Cretaceous bedded ironstones and shales exposed on a high ridge on the west side of Big Fish River, Yukon, Canada. The associated minerals include siderite, lazulite, an arrojadite-group mineral, kulanite, gormanite, quartz, and collinsite. Bobdownsite from the Yukon is tabular, colorless, and transparent, with a white streak and vitreous luster. It is brittle, with a Mohs hardness of ~5; no cleavage, parting, or macroscopic twinning is observed. The fracture is uneven and subconchoidal. The measured and calculated densities are 3.14 and 3.16 g/cm3, respectively. Bobdownsite is insoluble in water, acetone, or hydrochloric acid. Optically, it is uniaxial (–), v = 1.625(2), = 1.622(2). The electron-microprobe analysis yielded (Ca8.76Na0.24)S9.00 3+ 2+ (Mg0.72Fe 0.13Al0.11Fe 0.04)S1.00(P1.00O4)6(P1.00O3F1.07) as the empirical formula. Bobdownsite was examined with single-crystal X-ray diffraction; it is trigonal with space group R3c and unit-cell parameters a 10.3224(3), c 37.070(2) Å, V 3420.7(6) Å3. -

New Mineral Names*,†
American Mineralogist, Volume 106, pages 1537–1543, 2021 New Mineral Names*,† Dmitriy I. Belakovskiy1 and Yulia Uvarova2 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2CSIRO Mineral Resources, ARRC, 26 Dick Perry Avenue, Kensington, Western Australia 6151, Australia In this issue This New Mineral Names has entries for 11 new species, including bohuslavite, fanfaniite, ferrierite-NH4, feynmanite, hjalmarite, kenngottite, potassic-richterite, rockbridgeite-group minerals (ferrirockbridgeite and ferro- rockbridgeite), rudabányaite, and strontioperloffite. Bohuslavite* show a very strong and broad absorption in the O–H stretching region –1 D. Mauro, C. Biagoni, E. Bonaccorsi, U. Hålenius, M. Pasero, H. Skogby, (3600–3000 cm ) and a prominent band at 1630 (H–O–H bending) with F. Zaccarini, J. Sejkora, J. Plášil, A.R. Kampf, J. Filip, P. Novotný, a shoulder indicating two slightly different H2O environments in the –1 3+ structure. The weaker band at ~5100 cm is assigned to H2O combination R. Škoda, and T. Witzke (2019) Bohuslavite, Fe4 (PO4)3(SO4)(OH) mode (bending + stretching). The IR spectrum of bohuslavite from HM (H2O)10·nH2O, a new hydrated iron phosphate-sulfate. European Journal of Mineralogy, 31(5-6), 1033–1046. shows bands at: 3350, 3103, 1626, 1100, 977, 828, 750, 570, and 472 cm–1. Polarized optical absorption spectra show absorption bands due to 3+ 3+ electronic transitions in octahedrally coordinated Fe at 23 475, 22 000, Bohuslavite (2018-074a), ideally Fe4 (PO4)3(SO4)(OH)(H2O)10·nH2O, –1 triclinic, was discovered in two occurrences, in the Buca della Vena baryte and 18 250 cm . -

The Phosphate Mineral Arrojadite-(Kfe) and Its Spectroscopic Characteri- Zation
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Frost, Ray, Xi, Yunfei, Scholz, Ricardo, & Horta, Laura (2013) The phosphate mineral arrojadite-(KFe) and its spectroscopic characteri- zation. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 109, pp. 138-145. This file was downloaded from: https://eprints.qut.edu.au/58843/ c Consult author(s) regarding copyright matters This work is covered by copyright. Unless the document is being made available under a Creative Commons Licence, you must assume that re-use is limited to personal use and that permission from the copyright owner must be obtained for all other uses. If the docu- ment is available under a Creative Commons License (or other specified license) then refer to the Licence for details of permitted re-use. It is a condition of access that users recog- nise and abide by the legal requirements associated with these rights. If you believe that this work infringes copyright please provide details by email to [email protected] License: Creative Commons: Attribution-Noncommercial-No Derivative Works 2.5 Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1016/j.saa.2013.02.027 1 The phosphate mineral arrojadite-(KFe) and its spectroscopic characterization 2 3 Ray L.