OIICS Manual 2007 Section 2.3.3
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hydrogen and Carbon Isotope Fractionation During Degradation Of
ORIGINAL RESEARCH Hydrogen and carbon isotope fractionation during degradation of chloromethane by methylotrophic bacteria Thierry Nadalig1, Markus Greule2, Francßoise Bringel1,Stephane Vuilleumier1 & Frank Keppler2 1Equipe Adaptations et Interactions Microbiennes dans l’Environnement, UMR 7156 Universite de Strasbourg - CNRS, 28 rue Goethe, Strasbourg, 67083, France 2Max Planck Institute for Chemistry, Hahn-Meitner-Weg 1, 55128 Mainz, Germany Keywords Abstract Carbon isotope fractionation, chloromethane biodegradation, hydrogen isotope Chloromethane (CH3Cl) is a widely studied volatile halocarbon involved in the fractionation, methylotrophic bacteria. destruction of ozone in the stratosphere. Nevertheless, its global budget still remains debated. Stable isotope analysis is a powerful tool to constrain fluxes Correspondence of chloromethane between various environmental compartments which involve Thierry Nadalig, Universite de Strasbourg, a multiplicity of sources and sinks, and both biotic and abiotic processes. In UMR 7156 UdS-CNRS, 28 rue Goethe, this study, we measured hydrogen and carbon isotope fractionation of the 67083 Strasbourg Cedex, France. Tel: +33 3 68851973; Fax: +33 3 68852028; remaining untransformed chloromethane following its degradation by methylo- Methylobacterium extorquens Hyphomicrobium E-mail: [email protected] trophic bacterial strains CM4 and sp. MC1, which belong to different genera but both use the cmu pathway, the Funding Information only pathway for bacterial degradation of chloromethane characterized so far. Financial support for the acquisition of Hydrogen isotope fractionation for degradation of chloromethane was deter- GC-FID equipment by REALISE (http://realise. mined for the first time, and yielded enrichment factors (e)ofÀ29& and unistra.fr), the Alsace network for research À27& for strains CM4 and MC1, respectively. In agreement with previous and engineering in environmental sciences, is studies, enrichment in 13C of untransformed CH Cl was also observed, and gratefully acknowledged. -
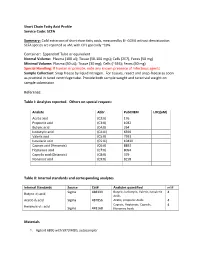
Short Chain Fatty Acid Profile Service Code: SCFA
Short Chain Fatty Acid Profile Service Code: SCFA Summary: Cold extraction of short chain fatty acids, measured by EI- GCMS without derivatization. SCFA species are reported as uM, with CV's generally ~10%. Container: Eppendorf Tube or equivalent Normal Volume: Plasma (100 ul); Tissue (50-100 mgs); Cells (2E7), Feces (50 mg) Minimal Volume: Plasma (50 uL); Tissue (30 mg); Cells (~5E6); Feces (40 mg) Special Handling: If human or primate, note any known presence of infectious agents. Sample Collection: Snap freeze by liquid nitrogen. For tissues, resect and snap-freeze as soon as practical in tared centrifuge tube. Provide both sample weight and tared vial weight on sample submission Reference: Table I: Analytes reported. Others on special request: Analyte Abbr. PubCHEM LOQ(uM) Acetic acid (C2:0) 176 Propionic acid (C3:0) 1032 Butyric acid (C4:0) 264 Isobutyric acid (C4:0i) 6590 Valeric acid (C5:0) 7991 Isovaleric acid (C5:0i) 10430 Caproic acid (Hexanoic) (C6:0) 8892 Heptanoic acid (C7:0) 8094 Caprylic acid (Octanoic) (C8:0) 379 Nonanoic acid (C9:0) 8158 Table II: Internal standards and corresponding analytes Internal Standards Source Cat# Analytes quantified mM Sigma 488399 Butyric, Isobutyric, Valeric, Isovaleric 4 Butyric-d7 acid Acids Acetic-d3 acid Sigma 487856 Acetic, propionic Acids 4 Caproic, Heptanoic, Caprylic, 4 Hexanoic-d11 acid Sigma 448168 Nonanoic Acids Materials 1. Agilent 6890 with 5973 MSD, autosampler 2. Vortexer 3. Refrigerated centrifuge, capable of 13,000g with eppendorf tube compatible rotor 4. ice bucket, ice 5. Balance 6. Prepared stock solutions of short chain fatty acid standards and isotope-labeled short chain fatty acid internal standards. -

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

540.14Pri.Pdf
Index Element names, parent hydride names and systematic names derived using any of the nomenclature systems described in this book are, with very few exceptions, not included explicitly in this index. If a name or term is referred to in several places in the book, the most informative references appear in bold type, and some of the less informative places are not cited in the index. Endings and suffixes are represented using a hyphen in the usual fashion, e.g. -01, and are indexed at the place where they would appear ignoring the hyphen. Names of compounds or groups not included in the index may be found in Tables P7 (p. 205), P9 (p. 232) and PIO (p. 234). ~, 3,87 acac, 93 *, 95 -acene, 66 \ +, 7,106 acetals, 160-161 - (minus), 7, 106 acetate, 45 - (en dash), 124-126 acetic acid, 45, 78 - (em dash), 41, 91, 107, 115-116, 188 acetic anhydride, 83 --+, 161,169-170 acetoacetic acid, 73 ct, 139, 159, 162, 164, 167-168 acetone, 78 ~, 159, 164, 167-168 acetonitrile, 79 y, 164 acetyl, III, 160, 163 11, 105, 110, 114-115, 117, 119-128, 185 acetyl chloride, 83, 183 K, 98,104-106,117,120,124-125, 185 acetylene, 78 A, 59, 130 acetylide, 41 11, 89-90,98, 104, 107, 113-116, 125-126, 146-147, acid anhydrides, see anhydrides 154, 185 acid halides, 75,83, 182-183 TC, 119 acid hydrogen, 16 cr, 119 acids ~, 167 amino acids, 25, 162-163 00, 139 carboxylic acids, 19,72-73,75--80, 165 fatty acids, 165 A sulfonic acids, 75 ct, 139,159,162,164,167-168 see also at single compounds A, 33-34 acrylic acid, 73, 78 A Guide to IUPAC Nomenclature of Organic actinide, 231 Compounds, 4, 36, 195 actinoids (vs. -

Federal Register/Vol. 81, No. 250/Thursday, December 29, 2016
95886 Federal Register / Vol. 81, No. 250 / Thursday, December 29, 2016 / Rules and Regulations C. Regulatory Flexibility Act (RFA) I. National Technology Transfer and ENVIRONMENTAL PROTECTION Advancement Act (NTTAA) AGENCY This action is not subject to the RFA. This rulemaking does not involve The RFA applies only to rules subject to 40 CFR Part 180 notice-and-comment rulemaking technical standards. requirements under the APA, 5 U.S.C. [EPA–HQ–OPP–2016–0007 and EPA–HQ– J. Executive Order 12898: Federal OPP–2016–0008; FRL–9950–40] 553, or any other statute. This rule is not Actions To Address Environmental subject to notice-and-comment Justice in Minority Populations and Isobutyl Acetate and Isobutyric Acid; requirements because the agency has Low-Income Populations Exemption From the Requirement of a invoked the APA ‘‘good cause’’ The EPA believes that this action is Tolerance exemption under 5 U.S.C. 553(b). not subject to Executive Order 12898 (59 AGENCY: Environmental Protection FR 7629, February 16, 1994) because it D. Unfunded Mandates Reform Act Agency (EPA). (UMRA) does not establish an environmental health or safety standard. This good ACTION: Final rule. This action does not contain any cause final action simply extends the SUMMARY: This regulation establishes unfunded mandate of $100 million or date for the EPA to take action on a more as described in UMRA, 2 U.S.C. exemptions from the requirement of a petition and does not have any impact tolerance for residues of isobutyl acetate 1531–1538, and does not significantly or on human health or the environment. -

Organophosphate Poisoning : a Review
120 Sinha and Sharma Med J Indones Organophosphate poisoning : A review Parmod K. Sinha, Ashok Sharma Abstrak Pestisida organofosfat digunakan secara luas di seluruh dunia. Keracunan oleh bahan ini merupakan masalah kesehatan masyarakat, terutama di negara berkembang. Zat neurotoksik organofosfat merupakan bahan yang dianggap mengancam dalam bidang militer dan terorisme. Mekanisme toksisitas bahan ini adalah dengan cara menghambat asetilkolinesterase yang mengakibatkan menumpuknya neurotransmitor asetilkolin dan terjadi rangsangan terus-menerus pada reseptor asetilkolin pada sistem saraf sentral maupun perifer. Selain krisis kolinergik, organofosfat dapat menimbulkan berbagai sindrom neurologis, baik akut maupun kronik. Sedangkan gejala peralihan ( intermediate) terjadi 1-4 hari setelah krisis kolinergik teratasi. Pengobatan standar terdiri dari reaktivasi asetilkolinesterase dengan antidot golongan oksim (prolidoksim, oksidoksime, HI-6 dan HLo7), dan pengendalian efek biokimia asetilkolin dengan menggunakan atropin. Golongan oksim yang baru HI-6 dan Hlo7 merupakan reaktivator asetilkolinesterase yang lebih cocok dan efektif untuk keracunan akut dan berat dibandingkan dengan prolidoksim dan obidoksim. Penderita yang mendapat pengobatan segera, biasanya dapat sembuh dari toksisitas akut, namun gejala neurologis ikutan dapat saja terjadi. (Med J Indones 2003; 12: 120-6) Abstract Organophosphate pesticides are used extensively worldwide, and poisoning by these agents, particularly in developing nations is a public health problem. Organophosphorous -

Guide No. 1 – October 2020 2/12 the CONCEPT and IMPLEMENTATION of CPA GUIDANCE RESIDUE LEVELS
Cooperation Centre for Scientific Research Relative to Tobacco CORESTA GUIDE N° 1 The Concept and Implementation of CPA Guidance Residue Levels October 2020 Agro-Chemical Advisory Committee CORESTA TECHNICAL GUIDE N° 1 Title: The Concept and Implementation of CPA Guidance Residue Levels Status: Valid Note: This document will be periodically reviewed by CORESTA Document history: Date of review Information July 2003 Version 1 GRL for Pyrethrins () and Terbufos corrected. December 2003 CPA terminology corrected. June 2008 Version 2 – GRLs revised and residue definitions added Provisional GRL of 2.00 ppm for Cyfluthrin to replace previous June 2010 GRL of 0.50 ppm July 2013 Version 3 – GRLs revised October 2013 Note for Maleic Hydrazide revised Version 4 – GRLs revised + clarification that scope of GRLs July 2016 applies predominantly to the production of traditional cigarette tobaccos and GAP associated with their cultivation. June 2018 Fluopyram GRL of 5 ppm added to GRL list Version 5 – Nine new CPAs with GRL added to list. November 2019 Revision of GRLs for Chlorantraniliprole and Indoxacarb. Updated web links. October 2020 Version 6 – Flupyradifurone GRL of 21 ppm added to GRL list. CORESTA Guide No. 1 – October 2020 2/12 THE CONCEPT AND IMPLEMENTATION OF CPA GUIDANCE RESIDUE LEVELS Executive Summary • Guidance Residue Levels (GRLs) are in the remit of the Agro-Chemical Advisory Committee (ACAC) of CORESTA. Their development is a joint activity of all ACAC members, who represent the leaf production, processing and manufacturing sectors of the Tobacco Industry. The concept of GRLs and their implementation are described in this guide. • GRLs provide guidance to tobacco growers and assist with interpretation and evaluation of results from analyses of residues of Crop Protection Agents (CPAs*). -

Periodic Trends in the Main Group Elements
Chemistry of The Main Group Elements 1. Hydrogen Hydrogen is the most abundant element in the universe, but it accounts for less than 1% (by mass) in the Earth’s crust. It is the third most abundant element in the living system. There are three naturally occurring isotopes of hydrogen: hydrogen (1H) - the most abundant isotope, deuterium (2H), and tritium 3 ( H) which is radioactive. Most of hydrogen occurs as H2O, hydrocarbon, and biological compounds. Hydrogen is a colorless gas with m.p. = -259oC (14 K) and b.p. = -253oC (20 K). Hydrogen is placed in Group 1A (1), together with alkali metals, because of its single electron in the valence shell and its common oxidation state of +1. However, it is physically and chemically different from any of the alkali metals. Hydrogen reacts with reactive metals (such as those of Group 1A and 2A) to for metal hydrides, where hydrogen is the anion with a “-1” charge. Because of this hydrogen may also be placed in Group 7A (17) together with the halogens. Like other nonmetals, hydrogen has a relatively high ionization energy (I.E. = 1311 kJ/mol), and its electronegativity is 2.1 (twice as high as those of alkali metals). Reactions of Hydrogen with Reactive Metals to form Salt like Hydrides Hydrogen reacts with reactive metals to form ionic (salt like) hydrides: 2Li(s) + H2(g) 2LiH(s); Ca(s) + H2(g) CaH2(s); The hydrides are very reactive and act as a strong base. It reacts violently with water to produce hydrogen gas: NaH(s) + H2O(l) NaOH(aq) + H2(g); It is also a strong reducing agent and is used to reduce TiCl4 to titanium metal: TiCl4(l) + 4LiH(s) Ti(s) + 4LiCl(s) + 2H2(g) Reactions of Hydrogen with Nonmetals Hydrogen reacts with nonmetals to form covalent compounds such as HF, HCl, HBr, HI, H2O, H2S, NH3, CH4, and other organic and biological compounds. -

Substitution and Redox Chemistry of Ruthenium Complexes
iJ il r¿ SUBSTITUTION AND REDOX CHEMISTRY OF RUTHENIUM COMPLEXES by Paul Stuaft Moritz, B. Sc. (Hons) * A Thesis submitted for the Degree of Doctor of Philosophy. The Department of Physical and lnorganic Chemistry, The University of Adelaide. JUNE 1987 lìr.+:-c{,¡l I /tz lZ]' STATEMENT. This Thesis conta¡ns no materialwhich has been accepted for the award of any other Degree or Diploma in any University and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference is made in the text. I give consent that, if this Thesis is accepted for the award of the Degree of Doctor of Philosophy, it may be made available for photocopying and, if applicable, loan. Moritz. SUMMARY The coordination chemislry of ruthenium is domínated by the oxidation states, +2 and +3. Within these oxidation states, the ammine complexes form a large and welt- characterized group. This thesis reports on the chemistry of lhe hitherto neglected triammine complexes, with particular reference to their redox chemistry, and the possible formation of Ru(lV) triammine complexes with terminal oxo ligands. The chemistry of the +4 oxidation state is further explored through the formation of stable chelate complexes. The salt, [Ru(NH3)3(OH2)31(CFgSO3)3, was prepared by hydrotysis of Ru(NHs)sCts in triflic acid solution. lts spectra, electrochemistry and substitution reactions are similar to those of the well-known hexa-, penta-, and tetraammine complexes. At freshly polished platinum and glassy carbon electrodes, a quasi reversible redox wave was detected, corresponding to a proton-coupled reduction involving the pu3+7pu2+ couple. -

Download Author Version (PDF)
PCCP Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/pccp Page 1 of 24 Physical Chemistry Chemical Physics Disulfuric acid dissociated by two water molecules: Ab initio and density functional theory calculations Seong Kyu Kim, 1,* Han Myoung Lee, 2 Kwang S. Kim 2,* 1 Department of Chemistry, Sungkyunkwan University, Suwon 440-746, Korea Manuscript 2Center for Superfunctional Materials, Department of Chemistry, Ulsan Institute of Science and Technology (UNIST), Ulsan 689-798, Korea *Corresponding authors, [email protected] , [email protected] Accepted Abstract We have studied geometries, energies and vibrational spectra of disulfuric acid - (H 2S2O7) and its anion (HS 2O7 ) hydrated by a few water molecules, using density functional theory (M062X) and ab initio theory (SCS-MP2 and CCSD(T)). -

Step-By-Step Guide to Better Laboratory Management Practices
Step-by-Step Guide to Better Laboratory Management Practices Prepared by The Washington State Department of Ecology Hazardous Waste and Toxics Reduction Program Publication No. 97- 431 Revised January 2003 Printed on recycled paper For additional copies of this document, contact: Department of Ecology Publications Distribution Center PO Box 47600 Olympia, WA 98504-7600 (360) 407-7472 or 1 (800) 633-7585 or contact your regional office: Department of Ecology’s Regional Offices (425) 649-7000 (509) 575-2490 (509) 329-3400 (360) 407-6300 The Department of Ecology is an equal opportunity agency and does not discriminate on the basis of race, creed, color, disability, age, religion, national origin, sex, marital status, disabled veteran’s status, Vietnam Era veteran’s status or sexual orientation. If you have special accommodation needs, or require this document in an alternate format, contact the Hazardous Waste and Toxics Reduction Program at (360)407-6700 (voice) or 711 or (800) 833-6388 (TTY). Table of Contents Introduction ....................................................................................................................................iii Section 1 Laboratory Hazardous Waste Management ...........................................................1 Designating Dangerous Waste................................................................................................1 Counting Wastes .......................................................................................................................8 Treatment by Generator...........................................................................................................12 -

CHEM 301 Assignment #2 Provide Solutions to the Following Questions in a Neat and Well-Organized Manner
CHEM 301 Assignment #2 Provide solutions to the following questions in a neat and well-organized manner. Reference data sources for any constants and state assumptions, if any. Due date: Thursday, Oct 20th, 2016 Attempt all questions. Only even numbers will be assessed. 1. A pond in an area affected by acid mine drainage is observed to have freshly precipitated Fe(OH)3(s) at pH 4. What is the minimum value of pe in this water? Use the appropriate pe-pH speciation diagram to predict the dominant chemical speciation of carbon, sulfur and copper under these conditions. 2. The solubility of FeS(s) in marine sediments is expected to be affected by the pH and the [H2S(aq)] in the surrounding pore water? Calculate the equilibrium concentration of Fe2+ (ppb) at pH 5 and pH 8, -3 assuming the H2S is 1.0 x 10 M. pKsp(FeS) = 16.84. + 2+ FeS(s) + 2 H (aq) ==== Fe (aq) + H2S 3. Boric acid is a triprotic acid (H3BO3); pKa1 = 9.24, pKa2 = 12.74 and pKa3 = 13.80. a) Derive an expression for the fractional abundance of H3BO3 as a function of pH b) Construct a fully labeled pH speciation diagram for boric acid over the pH range of 0 to 14 using Excel spreadsheet at a 0.2 pH unit interval. 4. At pCl- = 2.0, Cd2+ and CdCl+ are the only dominant cadmium chloride species with a 50% fractional abundance of each. + a) Calculate the equilibrium constant for the formation of CdCl (1). + - b) Derive an expression for the fractional abundance of CdCl as a function of [Cl ] and 1 – 4 for cadmium chloro species.