Cerebrospinal Fluid Analysis in Multiple Sclerosis Diagnosis: an Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Practice Guideline: Disease-Modifying Therapies for Adults with Multiple Sclerosis
Practice guideline: Disease-modifying therapies for adults with multiple sclerosis Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology Alexander Rae-Grant, MD1; Gregory S. Day, MD, MSc2; Ruth Ann Marrie, MD, PhD3; Alejandro Rabinstein, MD4; Bruce A.C. Cree, MD, PhD, MAS5; Gary S. Gronseth, MD6; Michael Haboubi, DO7; June Halper, MSN, APN-C, MSCN8; Jonathan P. Hosey, MD9; David E. Jones, MD10; Robert Lisak, MD11; Daniel Pelletier, MD12; Sonja Potrebic, MD, PhD13; Cynthia Sitcov14; Rick Sommers, LMSW15; Julie Stachowiak, PhD16; Thomas S.D. Getchius17; Shannon A. Merillat, MLIS18; Tamara Pringsheim, MD, MSc19 1. Department of Neurology, Cleveland Clinic, OH 2. Department of Neurology, Charles F. and Joanne Knight Alzheimer Disease Research Center, Washington University in St. Louis, MO 3. Departments of Medicine and Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada 4. Department of Neurology, Mayo Clinic, Rochester, MN 5. UCSF Weill Institute for Neurosciences, Department of Neurology, University of California, San Francisco 6. Department of Neurology, Kansas University Medical Center, Kansas City 7. Department of Neurology, School of Medicine, University of Louisville, KY 8. Consortium of Multiple Sclerosis Centers, Hackensack, NJ 9. Department of Neuroscience, St. Luke’s University Health Network, Bethlehem, PA 10. Department of Neurology, School of Medicine, University of Virginia, Charlottesville 11. Consortium of Multiple Sclerosis Centers, Hackensack, NJ, and Department of Neurology, School of Medicine, Wayne State University, Detroit, MI 12. Department of Neurology, Keck School of Medicine, University of Southern California, Los Angeles 13. Neurology Department, Southern California Permanente Medical Group, Kaiser, Los Angeles 14. -

Vaccination and Demyelination: Is There a Link? Examples with Anti
Vaccination and demyelination : Is there a link? Examples with anti-hepatitis B and papillomavirus vaccines Julie Mouchet Le Moal To cite this version: Julie Mouchet Le Moal. Vaccination and demyelination : Is there a link? Examples with anti-hepatitis B and papillomavirus vaccines. Human health and pathology. Université de Bordeaux, 2019. English. NNT : 2019BORD0015. tel-02134582 HAL Id: tel-02134582 https://tel.archives-ouvertes.fr/tel-02134582 Submitted on 20 May 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. THÈSE PRÉSENTÉE POUR OBTENIR LE GRADE DE DOCTEUR DE L’UNIVERSITÉ DE BORDEAUX ÉCOLE DOCTORALE : Sociétés, Politique, Santé Publique (SP2) SPÉCIALITÉ : Pharmacologie option Pharmaco-épidémiologie, Pharmacovigilance Par Julie MOUCHET LE MOAL VACCINATION ET RISQUE DE DEMYELINISATION : EXISTE-T-IL UN LIEN ? EXEMPLES DES VACCINS ANTI-HEPATITE B ET ANTI-PAPILLOMAVIRUS Sous la direction de : Monsieur le Professeur Bernard Bégaud Soutenue publiquement le 29 Janvier 2019 Composition du jury Président : Christophe TZOURIO, Professeur des Universités -

Clinical and MRI Clues and Pitfalls in the Diagnosis and Differential Diagnosis of Multiple Sclerosis
Clinical and MRI clues and pitfalls in the diagnosis and differential diagnosis of Multiple Sclerosis Aksel Siva, M.D. MS Clinic & Department Of Neurology Istanbul University Cerrahpaşa School of Medicine [email protected] MSParis2017 - 7th Joint ECTRIMS - ACTRIMS Meeting, 25 - 28 October 2017 Disclosure • Received research grants to my department from The Scientific and Technological Research Council Of Turkey - Health Sciences Research Grants numbers : 109S070 and 112S052.; and also unrestricted research grants from Merck-Serono and Novartis to our Clinical Neuroimmunology Unit • Honoraria or consultation fees and/or travel and registration coverage for attending several national or international congresses or symposia, from Merck Serono, Biogen Idec/Gen Pharma of Turkey, Novartis, Genzyme, Roche and Teva. • Educational presentations at programmes & symposia prepared by Excemed internationally and at national meetings and symposia sponsored by Bayer- Schering AG; Merck-Serono;. Novartis, Genzyme and Teva-Turkey; Biogen Idec/Gen Pharma of Turkey Introduction… • The incidence and prevalence rates of MS are increasing, so are the number of misdiagnosed cases as MS! • One major source of misdiagnosis is misinterpretation of nonspecific clinical and imaging findings and misapplication of MRI diagnostic criteria resulting in an overdiagnosis of MS! • The differential diagnosis of MS includes the MS spectrum and related disorders that covers subclinical & clinical MS phenotypes, MS variants and inflammatory astrocytopathies, as well as other Ab-associated atypical inflammatory-demyelinating syndromes • There are a number of systemic diseases in which either the clinical or MRI findings or both may mimic MS, which further cause confusion! Related publication *Siva A. Common Clinical and Imaging Conditions Misdiagnosed as Multiple Sclerosis. -

Serial Magnetic Resonance Imaging Changes of Pseudotumor Lesions In
Yan et al. BMC Neurol (2021) 21:219 https://doi.org/10.1186/s12883-021-02250-4 CASE REPORT Open Access Serial magnetic resonance imaging changes of pseudotumor lesions in retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations: a case report Yuying Yan1, Shuai Jiang1, Ruilin Wang2, Xiang Wang3, Peng Li3* and Bo Wu1* Abstract Background: Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations (RVCL-S) is an adult-onset rare monogenic microvasculopathy. Its typical neuroimaging features are punctate white matter lesions or pseudotumor alterations. RVCL-S is often under-recognized and misdiagnosed because of its rarity and similar imaging manifestations to multiple sclerosis or brain malignant mass. Case presentation: Here we report a case of a 36-year-old Chinese man who developed multiple tumefactive brain lesions spanning over two years leading to motor aphasia, cognitive decline, and limb weakness. He also presented with slight vision loss, and fundus fuorescein angiography indicated retinal vasculopathy. He underwent brain biopsies twice and showed no evidence of malignancy. Given the family history that his father died of a brain mass of unclear etiology, RVCL-S was suspected, and genetic analysis confrmed the diagnosis with a heterozygous insertion mutation in the three-prime repair exonuclease 1 gene. He was given courses of corticosteroids and cyclophospha- mide but received little response. Conclusions: The present case is one of the few published reports of RVCL-S with two-year detailed imaging data. Serial magnetic resonance images showed the progression pattern of the lesions. Our experience emphasizes that a better understanding of RVCL-S and considering it as a diferential diagnosis in patients with tumefactive brain lesions may help avoid unnecessary invasive examinations and make an earlier diagnosis. -

MR Images, Brain Lesions, and Deep Learning
applied sciences Review MR Images, Brain Lesions, and Deep Learning Darwin Castillo 1,2,3,* , Vasudevan Lakshminarayanan 2,4 and María José Rodríguez-Álvarez 3 1 Departamento de Química y Ciencias Exactas, Sección Fisicoquímica y Matemáticas, Universidad Técnica Particular de Loja, San Cayetano Alto s/n, Loja 11-01-608, Ecuador 2 Theoretical and Experimental Epistemology Lab, School of Optometry and Vision Science, University of Waterloo, Waterloo, ON N2L3G1, Canada; [email protected] 3 Instituto de Instrumentación para Imagen Molecular (i3M) Universitat Politècnica de València—Consejo Superior de Investigaciones Científicas (CSIC), E-46022 Valencia, Spain; [email protected] 4 Departments of Physics, Electrical and Computer Engineering and Systems Design Engineering, University of Waterloo, Waterloo, ON N2L3G1, Canada * Correspondence: [email protected]; Tel.: +593-07-370-1444 (ext. 3204) Featured Application: This review provides a critical review of deep/machine learning algo- rithms used in the identification of ischemic stroke and demyelinating brain diseases. It eval- uates their strengths and weaknesses when applied to real world clinical data. Abstract: Medical brain image analysis is a necessary step in computer-assisted/computer-aided diagnosis (CAD) systems. Advancements in both hardware and software in the past few years have led to improved segmentation and classification of various diseases. In the present work, we review the published literature on systems and algorithms that allow for classification, identification, and detection of white matter hyperintensities (WMHs) of brain magnetic resonance (MR) images, specifically in cases of ischemic stroke and demyelinating diseases. For the selection criteria, we used bibliometric networks. Of a total of 140 documents, we selected 38 articles that deal with the main objectives of this study. -

OLIGOCLONAL Igg BANDS in the CEREBROSPINAL FLUID of PORTUGUESE PATIENTS with MULTIPLE SCLEROSIS
Arq Neuropsiquiatr 2005;63(2-B):375-379 OLIGOCLONAL IgG BANDS IN THE CEREBROSPINAL FLUID OF PORTUGUESE PATIENTS WITH MULTIPLE SCLEROSIS Negative results indicate benign disease Maria José Sá1, Lucinda Sequeira2, Maria Edite Rio3, Edward J. Thompson4 ABSTRACT - We assessed the frequency of cerebrospinal fluid (CSF) restricted oligoclonal IgG bands (IgG- OCB) in Portuguese multiple sclerosis (MS) patients and its relationship with outcome. Paired CSF/serum samples of 406 patients with neurological disorders were submitted to isoelectric focusing with immunodetection of IgG. Ninety-two patients had definite MS; non-MS cases were assembled in groups inflammatory/infectious diseases (ID, n=141) and other/controls (OD, n=173). We found in the MS group: mean duration, 38.9 months; clinically isolated syndromes, 24%; relapsing/remitting course (RR), 65%; in RR patients the mean EDSS was 2.1 and the mean index of pro g ression was 0.31. Positive patterns significantly p redominated in MS (82.6%; ID, 40.4%; OD, 3.5%). The sensitivity and the specificity of positive IgG-OCB for MS diagnosis was 82.6% and 79.9%, respectively. The sole statistically significant difference in the MS g roup was the lower pro g ression index observed in negative cases. We conclude that the frequency of positive IgG-OCB patterns in our MS patients fits most values reported in the literature, and that negative results indicate benign disease. KEY WORDS: multiple sclerosis, CSF, oligoclonal bands, IgG, isoelectric focusing. Bandas oligoclonais da IgG no líquido céfalo-raquidiano de doentes portugueses com esclerose múltipla: resultados negativos indicam doença benigna RESUMO - Analisamos a frequência de bandas oligoclonais (BOC) restritas ao líquido céfalo-raquidiano (LCR) em doentes portugueses com esclerose múltipla (EM) e sua relação com a clínica. -

Demyelination: What Is It and Why Does It Happen?
Demyelination: What Is It and Why Does It Happen? • Causes • Symptoms • Types • MS and Demyelination • Treatment and Diagnosis • Vaccines • Takeaway What is demyelination? Nerves send and receive messages from every part of your body and process them in your brain. Nerves allow you to speak, see, feel, and think. Many nerves are coated in myelin. Myelin is an insulating material. When it’s worn away or damaged, nerves can deteriorate, causing problems in the brain and throughout the body. Damage to myelin around nerves is called demyelination. Nerves Nerves are made up of neurons. Neurons are composed of a cell body, dendrites, and an axon. The axon sends messages from one neuron to the next. The axon also connects neurons to other cells, such as muscle cells. Some axons are extremely short. Others are 3 feet long. Some axons are covered in myelin. Myelin protects the axons and helps carry axon messages as quickly as possible. Myelin Myelin is made of membrane layers that cover an axon. This is similar to the idea of an electrical wire with coating to protect the metal underneath. Myelin allows a nerve signal to travel faster. In unmyelinated neurons, a signal can travel along the nerves at about 1 meter per second. In a myelinated neuron, the signal can travel 100 meters per second. Certain diseases can damage myelin. Demyelination slows down messages sent along axons and causes the axon to deteriorate. Depending upon the location of the damage, axon loss can cause problems with feeling, moving, seeing, hearing, and thinking clearly. CAUSES Causes of demyelination Inflammation is the most common cause of myelin damage. -
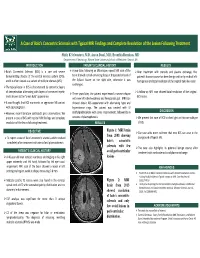
A Case of Balo's Concentric Sclerosis with Typical MRI Findings and Complete Resolution of the Lesion Followi
A Case of Balo’s Concentric Sclerosis with Typical MRI Findings and Complete Resolution of the Lesion Following Treatment Shitiz K Sriwastava, M.D, Aaron Desai, M.D, Evanthia Bernitsas, MD Department of Neurology, Wayne State University School of Medicine, Detroit, MI INTRODUCTION PATEINT’S CLINCIAL HISTORY RESULTS Balo’s Concentric Sclerosis (BCS) is a rare and severe A year later, following an MS relapse, repeat MRI scan of the After treatment with steroids and plasma exchange, the demyelinating disease of the central nervous system (CNS), brain showed a small enhancing focus at the posterior level of patient’s disease course has been benign with only residual left and it is often viewed as a variant of multiple sclerosis (MS). the Sylvian fissure on the right side, otherwise it was hemiparesis and total resolution of the original Balo‐like lesion. unchanged. The typical lesion in BCS is characterized by concentric layers of demyelination alternating with layers of preserved myelin A follow‐up MRI scan showed total resolution of the original Three years later, the patient experienced a severe relapse and is known as the “onion bulb” appearance. BCS lesion. with new left‐sided weakness and hemiparetic gait. MRI scan It was thought that BCS represents an aggressive MS variant showed classic BCS appearance with alternating hypo and with poor prognosis. hyperintense rings. The patient was treated with IV DISCUSSION However, recent literature contradicts prior observations. We methylprednisolone with some improvement, followed by 6 present a case of BCS with typical MRI findings and complete sessions of plasmapheresis. We present this case of BCS to shed light on this rare subtype resolution of the lesion following treatment. -

New Insights Into Multiple Sclerosis Mechanisms: Lipids on the Track to Control Inflammation and Neurodegeneration
International Journal of Molecular Sciences Review New Insights into Multiple Sclerosis Mechanisms: Lipids on the Track to Control Inflammation and Neurodegeneration Maria Podbielska 1,2,* , Joan O’Keeffe 3 and Anna Pokryszko-Dragan 4 1 Department of Biochemistry & Molecular Biology, Medical University of South Carolina, Charleston, SC 29425, USA 2 Laboratory of Microbiome Immunobiology, Ludwik Hirszfeld Institute of Immunology & Experimental Therapy, Polish Academy of Sciences, 53-114 Wroclaw, Poland 3 Department of Analytical, Biopharmaceutical and Medical Sciences, School of Science & Computing, Galway-Mayo Institute of Technology, Galway, Ireland; [email protected] 4 Department of Neurology, Wroclaw Medical University, 50-556 Wroclaw, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-71-370-9912 Abstract: Multiple sclerosis (MS) is a central nervous system disease with complex pathogenesis, including two main processes: immune-mediated inflammatory demyelination and progressive degeneration with axonal loss. Despite recent progress in our understanding and management of MS, availability of sensitive and specific biomarkers for these both processes, as well as neuroprotec- tive therapeutic options targeted at progressive phase of disease, are still being sought. Given their abundance in the myelin sheath, lipids are believed to play a central role in underlying immunopatho- genesis in MS and seem to be a promising subject of investigation in this field. On the basis of our previous research and a review of the literature, we discuss the current understanding of lipid-related mechanisms involved in active relapse, remission, and progression of MS. These insights highlight Citation: Podbielska, M.; O’Keeffe, J.; potential usefulness of lipid markers in prediction or monitoring the course of MS, particularly in its Pokryszko-Dragan, A. -
Antibodies in Multiple Sclerosis Oligoclonal Bands Target Debris Ryan C
COMMENTARY COMMENTARY Antibodies in multiple sclerosis oligoclonal bands target debris Ryan C. Wingera,b and Scott S. Zamvila,b,1 IgG oligoclonal bands (OCB) are detected in the of antibodies identified in CSF to OCB by studying cerebrospinal fluid (CSF) of more than 90% of multiple whole CSF IgG or recombinant antibodies constructed sclerosis (MS) patients and are considered the immu- from rearranged Ig heavy- and light-chain genes in nological hallmark that supports MS diagnosis. OCB individual B cells. are not unique to MS but are also observed in chronic Dornmair and coworkers used a combination of CNS infections, where they target their causative agents new biochemical, proteomic, and transcriptomic ap- (1–3). However, the target specificities of the IgG within proaches (4, 14) to examine the specificity of anti- OCB in MS have remained a mystery. Identification bodies in MS OCB. Disulfide-linked IgG heavy- (IgH) of those antigens recognized by OCB antibodies is and IgG light- (IgL) chain complexes were purified from thought to hold fundamental clues to the pathogenesis single OCB spots using affinity chromatography and of MS. In a recent PNAS publication, Brändle et al. (4) two-dimensional gel electrophoresis. Those antibody provide evidence that OCB in MS target ubiquitous (IgH2IgL2) complexes were then analyzed by mass intracellular antigens released in cellular debris. spectrometry to generate patient-specific Ig pepti- In 1942, Kabat et al. (5) established the diagnostic domes. In parallel, IgH and IgL genes, including the value of quantitative determinations of CSF gamma unique complementarity-determining region 3, from globulins in clinical neurology, and particularly in CSF B cells isolated from the corresponding patient MS. -

INFLAMMATORY/POST-INFECTIOUS ENCEPHALOMYELITIS L Bennetto, N Scolding I22
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2003.034256 on 20 February 2004. Downloaded from INFLAMMATORY/POST-INFECTIOUS ENCEPHALOMYELITIS L Bennetto, N Scolding i22 J Neurol Neurosurg Psychiatry 2004;75(Suppl I):i22–i28. doi: 10.1136/jnnp.2003.034256 ost-infectious and inflammatory encephalomyelitis are broadly represented by the syn- drome acute disseminated encephalomyelitis (ADEM). ADEM forms one of several Pcategories of primary inflammatory demyelinating disorders of the central nervous system. Others include multiple sclerosis (MS), acute transverse myelitis, and Devic’s disease. It should be remembered that these are syndromically defined diseases at present. There are no diagnos- tic tests presently available short of brain biopsy, but future advances may reveal diagnostic biological markers of disease and in so doing dramatically advance the pathophysiological and therapeutic understanding of these conditions. A wholesale change in classification and nomenclature may well follow. In the meantime clinicians must remain keenly appreciative of subtle shades of grey: ADEM, first MS relapse, or ultimately benign clinically isolated syn- drome? The answers are relevant to prognosis and, more recently, selection of the correct therapeutic strategy. c ACUTE DISSEMINATED ENCEPHALOMYELITIS ADEM is an inflammatory demyelinating disorder of the central nervous system that is usually monophasic, but a relapsing variant distinct from MS—multiphasic disseminated encephalomyelitis (MDEM) is well-described. ADEM is predominantly, though by no means copyright. exclusively, a disease of children and in particular infants. Historically it includes post- infectious encephalomyelitis and post-vaccination encephalomyelitis. While these two syndromes were distinguished by their precipitant it was realised that clinically and pathologically they were very similar. -

KIRA ÅKERLUND: Environmental and Lifestyle Factors in Multiple Sclerosis
ANNALES UNIVERSITATIS TURKUENSIS UNIVERSITATIS ANNALES D 1465 D Kira Åkerlund Kira ENVIRONMENTAL AND LIFESTYLE FACTORS IN MULTIPLE SCLEROSIS With Emphasis on Vitamin D, EBV Infection, Smoking and Cancer Risk Kira Åkerlund Painosalama Oy, Turku, Finland 2020 Finland Turku, Oy, Painosalama ISBN 978-951-29-7962-2 (PRINT) – ISBN 978-951-29-7963-9 (PDF) TURUN YLIOPISTON JULKAISUJA ANNALES UNIVERSITATIS TURKUENSIS ISSN 0355-9483 (Print) SARJA - SER. D OSA - TOM. 1465 | MEDICA - ODONTOLOGICA | TURKU 2020 ISSN 2343-3213 (Online) ENVIRONMENTAL AND LIFESTYLE FACTORS IN MULTIPLE SCLEROSIS With Emphasis on Vitamin D, EBV Infection, Smoking and Cancer Risk Kira Åkerlund TURUN YLIOPISTON JULKAISUJA – ANNALES UNIVERSITATIS TURKUENSIS SARJA - SER. D OSA – TOM. 1465 | MEDICA – ODONTOLOGICA | TURKU 2020 University of Turku Faculty of Medicine Department of Neurology Doctoral Programme in Clinical Research Division of Clinical Neurosciences Turku University Hospital Supervised by Docent Merja Soilu-Hänninen Division of Clinical Neurosciences Turku University Hospital Turku, Finland Reviewed by Docent Mikko Kallela Docent Markus Färkkilä Helsinki University Hospital University of Helsinki Department of Neurology Helsinki, Finland University of Helsinki Helsinki, Finland Opponent Professor Anne Remes Unit of Clinical Neuroscience Neurology, University of Oulu Oulu, Finland The originality of this publication has been checked in accordance with the University of Turku quality assurance system using the Turnitin OriginalityCheck service. ISBN 978-951-29-7962-2 (PRINT) ISBN 978-951-29-7963-9 (PDF) ISSN 0355-9483 (Print) ISSN 2343-3213 (Online) Painosalama Oy, Turku, Finland 2020 To Rita, Viktor and Finn 3 UNIVERSITY OF TURKU Faculty of Medicine Department of Neurology KIRA ÅKERLUND: Environmental and lifestyle factors in multiple sclerosis with emphasis on vitamin D, EBV infection, smoking and cancer risk Doctoral Dissertation, 155 pp.