Baseline Concentration of Morbid Leachate in Well Water in Ankpa, Kogi State, Nigeria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urban Sprawl, Pattern and Measurement in Lokoja, Nigeria
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Research Papers in Economics Alabi M. O. URBAN SPRAWL, PATTERN AND MEASUREMENT IN LOKOJA, NIGERIA URBAN SPRAWL, PATTERN AND MEASUREMENT IN LOKOJA, NIGERIA Michael Oloyede ALABI Department of Geography and Planning, Kogi State University P.M. B. 1008, Anyigba, Nigeria ement [email protected] Abstract Lokoja have been experiencing a large influx of population from its surrounding regions, which had led to rapid growth and expansion that had left profound changes on the landscape in terms of land use and land cover. This study uses the GIS techniques and the application of Shannon’s entropy theory to measure the behavior of sprawl which is based on the notion that landscape entropy or disorganization increases with sprawl, analysis was carried out based on the integration of remote sensing and GIS, the measurement of entropy is devised based on the town location factors, distance from roads, to reveal and capture spatial patterns of urban sprawl. Then Entropy value for each zone revealed a high value, especially areas outside the core city area; like Felele, with the entropy of 0.3, Adankolo, 0.2 and Lokongoma, 0.2. These areas are evenly dispersed settlement, as one move away from the city core. Study shows a correlation of population densities and entropy values of 1987 and 2007, for areas like Felele ,Adankolo, and Lokongoma , which is indicative of spread over space , an evidence of sprawl. But as we go down the table the entropy values seem to tend towards zero. -

Nigeria's Constitution of 1999
PDF generated: 26 Aug 2021, 16:42 constituteproject.org Nigeria's Constitution of 1999 This complete constitution has been generated from excerpts of texts from the repository of the Comparative Constitutions Project, and distributed on constituteproject.org. constituteproject.org PDF generated: 26 Aug 2021, 16:42 Table of contents Preamble . 5 Chapter I: General Provisions . 5 Part I: Federal Republic of Nigeria . 5 Part II: Powers of the Federal Republic of Nigeria . 6 Chapter II: Fundamental Objectives and Directive Principles of State Policy . 13 Chapter III: Citizenship . 17 Chapter IV: Fundamental Rights . 20 Chapter V: The Legislature . 28 Part I: National Assembly . 28 A. Composition and Staff of National Assembly . 28 B. Procedure for Summoning and Dissolution of National Assembly . 29 C. Qualifications for Membership of National Assembly and Right of Attendance . 32 D. Elections to National Assembly . 35 E. Powers and Control over Public Funds . 36 Part II: House of Assembly of a State . 40 A. Composition and Staff of House of Assembly . 40 B. Procedure for Summoning and Dissolution of House of Assembly . 41 C. Qualification for Membership of House of Assembly and Right of Attendance . 43 D. Elections to a House of Assembly . 45 E. Powers and Control over Public Funds . 47 Chapter VI: The Executive . 50 Part I: Federal Executive . 50 A. The President of the Federation . 50 B. Establishment of Certain Federal Executive Bodies . 58 C. Public Revenue . 61 D. The Public Service of the Federation . 63 Part II: State Executive . 65 A. Governor of a State . 65 B. Establishment of Certain State Executive Bodies . -

World Bank Document
The Final Draft RAP Report for Agassa Gully Erosion Sites for NEWMAP, Kogi State. Public Disclosure Authorized FINAL REPORT RESETTLEMENT ACTION PLAN (RAP) FOR AGASSA EROSION SITE, OKENE LOCAL GOVERNMENT AREA Public Disclosure Authorized SUBMITTED TO Public Disclosure Authorized KOGI STATE NIGERIA EROSION AND WATERSHED MANAGEMENT PROJECT (KGS-NEWMAP) PLOT 247, TUNDE OGBEHA STREET, GRA, LOKOJA. Public Disclosure Authorized i The Final Draft RAP Report for Agassa Gully Erosion Sites for NEWMAP, Kogi State. RAP Basic Data/Information S/N Subject Data 1 Intervention Site Agassa Gully Erosion sub-project, Okene LGA, Kogi State 2 Need for RAP Resettlement of People Displaced by the Project/Work 3 Nature of Civil Works Stabilization or rehabilitation in and around Erosion Gully site - stone revetment to reclaim and protect road way and reinforcement of exposed soil surface to stop scouring action of flow velocity, extension of culvert structure from the Agassa Road into the gully, chute channel, stilling basin, apron and installation of rip-rap and gabions mattress at some areas. Zone of Impact 5m offset from the gully edge. 4 Benefit(s) of the Intervention Improved erosion management and gully rehabilitation with reduced loss of infrastructure including roads, houses, agricultural land and productivity, reduced siltation in rivers leading to less flooding, and the preservation of the water systems for improved access to domestic water supply. 5 Negative Impact and No. of PAPs A census to identify those that could be potentially affected and eligible for assistance has been carried out. However, Based on inventory, a total of 241 PAPs have been identified. -

Local Government Service Delivery in Nigeria: an Empirical Comparison of Government Efforts and the People’S Expectations
E-ISSN 2039-2117 Mediterranean Journal of Social Sciences Vol 4 No 6 ISSN 2039-9340 Published by MCSER-CEMAS-Sapienza University of Rome July 2013 Local Government Service Delivery in Nigeria: An Empirical Comparison of Government Efforts and the People’s Expectations Achimugu, Hassan Department of Public Administration, Kogi State University, Anyigba- Nigeria 08038132529. [email protected] Stephen, Makoji Roberts Salford University Business School, Greater Manchester, Uk [email protected] Agboni, Uyo Joy Department of Public Administration, Kogi State University, Anyigba- Nigeria Doi:10.5901/mjss.2013.v4n6p335 Abstract The under- performance of Nigeria’s 776 Local governments have continued to attract huge policy and research concerns in the last three decades. This, among other reasons is because huge amount of resources is committed to this vital tier of government annually from the federation account, with very little visible impact on the lives of the nation’s teaming rural population. This paper focuses on Ofu Local government in Kogi State – North Central –Nigeria. It evaluates how the local government has performed with regards to its internal workings and service delivery. The findings show that Ofu local government’s performance by all indices does not justify the financial resources it has received from the federation account in the period under evaluation. The reasons for this under performance ranges from corruption to slow and inefficient administrative processes but most important is the fact that the peoples input is left out in policy designs. Hence government interventions are significantly at variance with the people’s felt – need. The paper recommends among others, a bridge of gap driven by the active involvement of the people at all stages of programmes designed to offer them services. -

KOGI STATE GOVERNORSHIP ELECTION 2019 Brief
KOGI STATE GOVERNORSHIP ELECTION 2019 Brief 1 BACKGROUND The Kogi State Governorship election is scheduled to take place on Saturday, November 16, 2019. The election will be taking place simultaneously with the governorship elections in Bayelsa State. These governorship elections would be the first elections to be conducted by INEC post-2019 general elections. Kogi State, with a land area of 29,833 square kilometres, was carved out of Kwara and Benue states on August 27, 1991. Kogi is one of the states in the north-central zone of Nigeria. It is popularly called the confluence state due to the fact that the confluence of Rivers Niger and Benue occur there. There are three main ethnic groups in the state namely Igala, Ebira, and Okun; with the Igalas being the largest ethnic group. Lokoja is the state capital. Kogi State, with a population of 3,314,043 according to 2006 census, is the most centrally located of all the states of the federation. It shares common boundaries with Niger, Kwara and Nasarawa states as well as the Federal Capital Territory (FCT) to the north Benue and Enugu states to the East; Enugu and Anambra states to the south; and to the west by Ondo, Ekiti and Edo states. PRESENT DAY GOVERNMENT OF KOGI STATE The present Governor of Kogi is Alhaji Yahaya Bello and the Deputy Governor of the State is Edward Onoja (his former Chief of Staff), who was sworn into office in October 2019 fpllpowing the controversial impeachment of the former Deputy Governor, Simon Achuba. On 5th December 2015, Governor Yahaya Bello was declared the elected Governor of the State after a supplementary election was held to conclude the inconclusive election of Saturday, 22nd November 2015. -

Evaluation of Groundwater Potential of Crystalline Basement Area of Kogi State Polytechnic, Osara Campus, North-Central Nigeria Using Electrical Resistivity Method
Journal of Environment and Earth Science www.iiste.org ISSN 2224-3216 (Paper) ISSN 2225-0948 (Online) Vol. 3, No.9, 2013 Evaluation of Groundwater Potential of Crystalline Basement Area of Kogi State Polytechnic, Osara Campus, North-Central Nigeria using Electrical Resistivity Method 1* O.K. Musa, 2 D.A. Ogbodo 3 S.S. Jatto 4 E.A. Kudamnya *1 Department of Geology, Federal University Lokoja, P.M.B 1154, Kogi State; 2 Department of Mineral Resources Engineering, Kogi State Polytechnic, Lokoja, Nigeria; 3Centre for Atmospheric Research, (NASRDA), Abuja; 4Department of Geology, Ahmadu Bello University Zaria *E-mail of the corresponding author: [email protected] Abstract Hydrogeophysical study was carried out in the Basement Complex area of Kogi State Polytechnic, Osara Campus, to investigate the groundwater potential of the subsurface layers. Acute water shortage and challenges of abortive and non-functional open and closed wells has prompted this study on the development of the groundwater resources. This work involves using the electrical resistivity method to investigate concealed fractures within the area with the aim of delineating near surface/subsurface geological layers to determine the depths, thickness, structural trends and spatial distribution of basement rocks as potential sources of groundwater. A total of thirty six vertical electrical sounding (VES) using the Schlumberger configuration was employed for the study. The depth and resistivity of the subsurface layers were determined. The interpreted results of the geo electrical sections indicates the presence of four subsurface layers: top soil of thickness and resistivity values ranging from 0.2 – 5.0m and 169 – 3728 ohms meter, weathered basement ranging from 5 – 12m and 100 – 914 ohms meter, fractured basement ranging from 6.2 – 196 meters and 42 - 196 ohms meter the fresh rock bedrock ranging from infinity in thickness and 741 – 3691 ohms metres. -

Protopterus Annectens (OWEN) in IDAH AREA of RIVER NIGER, NIGERIA
Animal Research International (2010) 7(3): 1264 – 1266 1264 LENGTH-WEIGHT RELATIONSHIP AND CONDITION FACTOR OF Protopterus annectens (OWEN) IN IDAH AREA OF RIVER NIGER, NIGERIA ADEYEMI, Samuel Olusegun Department of Biological Sciences, Kogi State University, PMB 1008, Anyigba Email: [email protected] Phone: +234 8062221968 ABSTRACT A total of 62 samples of Protopterus annectens (Owen) were examined for this study from Idah area of River Niger between August and November 2008. The length-weight relationship calculated for species gave a b-value of 2.55 which is indicative of negative allometric growth. It attained a length of 59cm and weight of 397g. The condition factor varied from 0.23 to 0.76 with a mean of 0.39+0.08 and showed that the fish was well and in good environment for growth and survival. Keywords: Protopterus annectens, Allometric growth, Survival, Length-weight relationship, Condition INTRODUCTION factor of P. annectens in order to aid its management in the river. Fish found in tropical and sub-tropical water system experience frequency growth MATERIALS AND METHODS fluctuations due to changes in food composition, environmental variables and spawning Study Area: The study area is Idah area of conditions among others. Length-weight and River Niger in Idah Local Government Area of length-length relationships can be used to asses Kogi State, Nigeria. The river extends from the influence of these factors in fish. Kulbicki et Lokoja via Ajaokuta, Itobe to Idah. The river is al. (1993) and King (1996) reported that fish located on latitude 7007N and longitude 6044E. growth, mean weight at a given body length of The water temperature range between 220C and fish and the relative wellbeing in fish can be 310C, Idah has a tropical savannah climate with known through this relationship. -
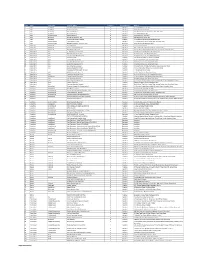
S/No State City/Town Provider Name Category Coverage Type Address
S/No State City/Town Provider Name Category Coverage Type Address 1 Abia AbaNorth John Okorie Memorial Hospital D Medical 12-14, Akabogu Street, Aba 2 Abia AbaNorth Springs Clinic, Aba D Medical 18, Scotland Crescent, Aba 3 Abia AbaSouth Simeone Hospital D Medical 2/4, Abagana Street, Umuocham, Aba, ABia State. 4 Abia AbaNorth Mendel Hospital D Medical 20, TENANT ROAD, ABA. 5 Abia UmuahiaNorth Obioma Hospital D Medical 21, School Road, Umuahia 6 Abia AbaNorth New Era Hospital Ltd, Aba D Medical 212/215 Azikiwe Road, Aba 7 Abia AbaNorth Living Word Mission Hospital D Medical 7, Umuocham Road, off Aba-Owerri Rd. Aba 8 Abia UmuahiaNorth Uche Medicare Clinic D Medical C 25 World Bank Housing Estate,Umuahia,Abia state 9 Abia UmuahiaSouth MEDPLUS LIMITED - Umuahia Abia C Pharmacy Shop 18, Shoprite Mall Abia State. 10 Adamawa YolaNorth Peace Hospital D Medical 2, Luggere Street, Yola 11 Adamawa YolaNorth Da'ama Specialist Hospital D Medical 70/72, Atiku Abubakar Road, Yola, Adamawa State. 12 Adamawa YolaSouth New Boshang Hospital D Medical Ngurore Road, Karewa G.R.A Extension, Jimeta Yola, Adamawa State. 13 Akwa Ibom Uyo St. Athanasius' Hospital,Ltd D Medical 1,Ufeh Street, Fed H/Estate, Abak Road, Uyo. 14 Akwa Ibom Uyo Mfonabasi Medical Centre D Medical 10, Gibbs Street, Uyo, Akwa Ibom State 15 Akwa Ibom Uyo Gateway Clinic And Maternity D Medical 15, Okon Essien Lane, Uyo, Akwa Ibom State. 16 Akwa Ibom Uyo Fulcare Hospital C Medical 15B, Ekpanya Street, Uyo Akwa Ibom State. 17 Akwa Ibom Uyo Unwana Family Hospital D Medical 16, Nkemba Street, Uyo, Akwa Ibom State 18 Akwa Ibom Uyo Good Health Specialist Clinic D Medical 26, Udobio Street, Uyo, Akwa Ibom State. -

For Agassa Gully Erosion Site, Okene Lga, Kogi State, Nigeria
THE NIGERIA EROSION AND WATERSHED MANAGEMENT PROJECT (NEWMAP) Public Disclosure Authorized Public Disclosure Authorized FINAL REPORT Public Disclosure Authorized OF ENVIRONMENTAL AND SOCIAL MANAGEMENT PLAN (ESMP) FOR AGASSA GULLY EROSION SITE, OKENE LGA, KOGI STATE, NIGERIA OCTOBER, 2018 Public Disclosure Authorized Environmental and Social Management Plan (ESMP) for Agassa Gully Erosion site in Okene LGA, Kogi State TABLE OF CONTENTS Page Table of Contents 1 List of Tables 6 List of Figures 7 List of Plates 7 Executive Summary 8 Abbreviations 19 CHAPTER ONE: INTRODUCTION 1.1 Background 22 1.2 Description of the Proposed Intervention 23 1.3 Environmental and Social Safeguard Concerns 24 1.4 Purpose of the ESMP 25 1.5 Objectives of the ESMP 26 1.6 Rationale for the study 27 1.7 Scope of Work 28 1.8 Technical Approach and Methodology 29 1.9 Study Approach 30 1.10 Literature/Data Review 30 1.11 Baseline Data Acquisition Methods 30 CHAPTER TWO: INSTITUTIONAL AND LEGAL FRAMEWORK FOR ENVIRONMENTAL MANAGEMENT 2.1 Introduction 36 2.2 National Regulatory Requirement 36 2.2.1 National Environmental Policy 36 2.2.2 National Environmental (Soil Erosion and Flood Control Regulations, 2001 by NESREA 36 2.2.3 Technical Guidelines on Soil Erosion, Flood and Coastal Zone Management 36 2.2.4 National Effluent Limitation Regulation 36 2.2.5 Pollution Abatement in Industries and Facilities Generating Wastes Regulation 37 2.2.6 Management of Hazardous and Solid Wastes Regulations 37 2.2.7 Environmental Impact Assessment Act 37 2.2.8 Land Use Act of 1978 37 -

The Intersection of China's Commercial Interests and Nigeria's
UNITED STATES INSTITUTE OF PEACE www.usip.org SPECIAL REPORT 2301 Constitution Ave., NW • Washington, DC 20037 • 202.457.1700 • fax 202.429.6063 About the Report Matthew T. Page This report examines how Chinese interests in Nigeria intersect with the country’s complicated conflict landscape, looking at both the constructive and the potentially destabilizing effects Chinese commercial activities have on Nigeria’s peace and security. The report was informed by fieldwork and The Intersection of interviews with Nigerian government officials and academics as well as international experts on China-Nigeria relations and Nigerian business leaders with extensive ties to local Chinese companies. The report was sponsored by the Asia Center’s China’s Commercial China program at the United States Institute of Peace. About the Author Interests and Nigeria’s Matthew T. Page is a consultant and co-author of Nigeria: What Everyone Needs to Know (Oxford University Press, 2018). An associate fellow with the Africa Programme at Chatham Conflict Landscape House, a nonresident scholar with the Carnegie Endowment for International Peace, and nonresident fellow with the Centre for Democracy and Development in Abuja, until recently he was the US intelligence community’s top Nigeria Summary expert, serving with the Department of State’s Bureau of • The foundation of China’s strategic interest in Nigeria is its robust commercial and Intelligence and Research, Defense Intelligence Agency, and investment footprint—especially the thriving bilateral trading relationship. Nigeria’s Marine Corps Intelligence. strategic importance as an export destination, source of oil outside the Middle East, and home to a growing consumer population means that it will continue to receive special attention from Beijing. -

Road Infrastructure and Urban Mobility in Selected Urban
ROAD INFRASTRUCTURE AND URBAN MOBILITY IN SELECTED URBAN CENTRES IN KOGI STATE, NIGERIA OLORUNFEMI, SAMUEL OLUWASEYI B.Tech; M.Tech (FUTA) TMT/03/2176 A Thesis in the Department of Transport Management Technology, School of Management Technology, submitted to the School of Postgraduate Studies in partial fulfilment of the requirements for the award of Dorctor of Philosophy (Ph.D) in Transport Management Technology of the Federal University of Technology, Akure, Nigeria. March, 2021 ABSTRACT This study assess road infrastructure and urban mobility in selected urban centres in Kogi State, Nigeria. The objectives of the research are to ascertain the spatial pattern of road infrastructure in the selected urban centres; investigate the satisfaction level of urban dwellers with the state of road transportation development; assess urban mobility challenges in the study area; and examine government investment in road transport. The study deployed questionnaire and field observation to collect the required data. Structured questionnaire were administered to one thousand, two hundred and fifteen (1,215) household heads in the study area to elicit information on their perception on the functionality of road infrastructure in the state with reference to their urban mobility. Descriptive statistics were expressed in percentages and weighted mean while inferential statistic deployed was Analysis of variance (ANOVA) which was adopted to test the hypothesis. ArcGIS 10 was deployed to produce the topological graph of all the selected urban centres: Adavi, Okene, Ankpa, Dekina, Mopa-Amuro, Kabba-Bunu, Lokoja, Ajaokuta and Bassa-Oguma being the urban centres where the nodes and links were derived. Road network and degree of connectivity were evaluated using topological graph derived from ArcGIS and analysed by Gamma index and cyclomatic index. -
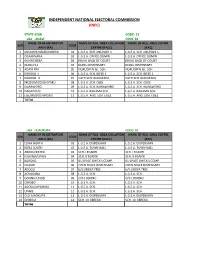
State Kogi Code: 23 Lga : Adavi Code: 01 Name of Registration Name of Reg
INDEPENDENT NATIONAL ELECTORAL COMMISSION (INEC) STATE KOGI CODE: 23 LGA : ADAVI CODE: 01 NAME OF REGISTRATION NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE AREA (RA) CENTRE (RACC) (RAC) 1 OKUNCHI/OZURI/ONIEKA 01 L.G.E.A. SCH. OKUNCHI 1 L.G.E.A. SCH. OKUNCHI 1 2 OGAMINANA 02 L.G.E.A. OFFICE OGMIN L.G.E.A. OFFICE OGMIN 3 IRUVUCHEBA 03 IRUVU BACK OF COURT IRUVU BACK OF COURT 4 IDANUHLI 04 IDANU DISPENSARY IDANU DISPENSARY 5 ADAVI EBA 05 NURUDEEN ISL. SCH. NURUDEEN ISL. SCH. 6 KUROKO -I 06 L.G.E.A. SCH. IBEKE 1 L.G.E.A. SCH. IBEKE 1 7 KUROKO -II 07 CATH SCH. IDAKATAPA CATH SCH. IDAKATAPA 8 INOZIOMI/OSISI/IPAKU 08 L.G.E.A. SCH. OSISI L.G.E.A. SCH. OSISI 9 IKARAWORO 09 L.G.E.A. SCH. IKARAWORO L.G.E.A. SCH. IKARAWORO 10 NAGAZI/F/C 10 L.G.E.A. RAHAMA SCH. L.G.E.A. RAHAMA SCH. 11 EGE/IRUVOCHINOMI 11 L.G.E.A. ANG. SCH. EGE1 L.G.E.A. ANG. SCH. EGE1 TOTAL LGA : AJAOKUTA CODE: 02 NAME OF REGISTRATION NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE AREA (RA) CENTRE (RACC) (RAC) 1 EBIYA NORTH 01 L.G.E.A. DISPENSARY L.G.E.A. DISPENSARY 2 EBIYA SOUTH 02 L.G.E.A. TOWN HALL L.G.E.A. TOWN HALL 3 ABODU/PATESI 03 SCH. I EGANYI SCH. I EGANYI 4 ICHUWA/UPAJA 04 SCH.