Morphology, Architecture and Growth of a Clonal Palm, Acoelorrhaphe Wrightii Sara M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aiphanes Grandis Borchs
Palm Conservation – Palm Specialist Group Aiphanes grandis Borchs. & Balslev Status: Endangered (EN) Common name None recorded Natural range Aiphanes grandis is endemic to the western slopes of the Andes mountains in Ecuador, at elevations between 1000 and 2000 m. It occurs in cloud forest in the central and southern parts of the country. Recognition characteristics It is a large, solitary palm tree, with a stem up to 20 m tall and 10–20 cm in diameter. The stem and leaves are fiercely armed with long, black spines. The leaf blade is 200–250 cm long with up to 50 leaflets on each side, briefly jagged at apex, inserted in groups and pointing in different directions. The inflorescence is 1–1.5 m long, with up to 200 spreading branches. Flowers are white to pale yellow and fruits 2–3 cm in diameter, dull green and covered with brown, loosely attached bristles. The combination of jagged leaflets and large size distinguishes the species from all other South American palms. Natural history Little is known about the natural history of the species. The palm heart is edible, and its seeds are sometimes pureed and cooked with crude cane sugar to form a nougat-like paste. Threats to survival The estimated loss of potential habitat for A. grandis, based on habitat modeling, is 62%, most of which has occurred over the last 30–40 years. The west Andean mountain forest in southern and central Ecudor, where A. grandis occurs, harbors a high number of endemic plant species. It is also the home to remnant populations of such conspicuous palm species as Ceroxylon ventricosum and C. -

Anatomia Foliar De Allagoptera Nees (Arecaceae) Como Subsídio À
Universidade de Brasília Instituto de Ciências Biológicas Departamento de Botânica Programa de Pós Graduação em Botânica Dissertação de Mestrado Anatomia foliar de Allagoptera Nees (Arecaceae) como subsídio à taxonomia André Silva Pinedo Orientadora: Profª Drª Sueli Maria Gomes Brasília, março de 2015 i Universidade de Brasília Instituto de Ciências Biológicas Departamento de Botânica Programa de Pós Graduação em Botânica Dissertação de Mestrado Anatomia foliar de Allagoptera Nees (Arecaceae) como subsídio à taxonomia Dissertação apresentada ao Programa de Pós- Graduação em Botânica da Universidade de Brasília como um dos requisitos para obtenção do título de Mestre em Botânica. André Silva Pinedo Orientadora: Profª Drª Sueli Maria Gomes Brasília, março de 2015 ii Universidade de Brasília Instituto de Ciências Biológicas Departamento de Botânica Programa de Pós Graduação em Botânica Banca examinadora: iii Agradecimentos Primeiramente a Deus, sou eternamente grato pela minha vida, pela oportunidade proporcionada e pela capacitação de estar realizando este curso de pós-graduação, que tem sido uma grande experiência de amadurecimento para mim; À minha orientadora, Profª. Drª. Sueli Maria Gomes, pela sua paciência ao me ensinar e constantes críticas construtivas que fez ao longo do período em que trabalhamos juntos, que fizeram desse mestrado uma grande oportunidade para o meu crescimento profissional; À Profª. Drª. Renata Corrêa Martins, por sua parceria nesse projeto, auxiliando na coleta e identificação das espécies, além de ser uma profissional extremamente prestativa; À Profª. Drª. Cássia Munhoz, minha orientadora em estágio de Iniciação Científica na graduação, motivando-me a trabalhar com plantas; Ao Prof. Dr. Eduardo Gomes Gonçalves, por ter me incentivado a trabalhar com o grupo das palmeiras; À Profª. -

Journal of the International Palm Society Vol. 58(1) Mar. 2014 the INTERNATIONAL PALM SOCIETY, INC
Palms Journal of the International Palm Society Vol. 58(1) Mar. 2014 THE INTERNATIONAL PALM SOCIETY, INC. The International Palm Society Palms (formerly PRINCIPES) Journal of The International Palm Society Founder: Dent Smith The International Palm Society is a nonprofit corporation An illustrated, peer-reviewed quarterly devoted to engaged in the study of palms. The society is inter- information about palms and published in March, national in scope with worldwide membership, and the June, September and December by The International formation of regional or local chapters affiliated with the Palm Society Inc., 9300 Sandstone St., Austin, TX international society is encouraged. Please address all 78737-1135 USA. inquiries regarding membership or information about Editors: John Dransfield, Herbarium, Royal Botanic the society to The International Palm Society Inc., 9300 Gardens, Kew, Richmond, Surrey, TW9 3AE, United Sandstone St., Austin, TX 78737-1135 USA, or by e-mail Kingdom, e-mail [email protected], tel. 44-20- to [email protected], fax 512-607-6468. 8332-5225, Fax 44-20-8332-5278. OFFICERS: Scott Zona, Dept. of Biological Sciences (OE 167), Florida International University, 11200 SW 8 Street, President: Leland Lai, 21480 Colina Drive, Topanga, Miami, Florida 33199 USA, e-mail [email protected], tel. California 90290 USA, e-mail [email protected], 1-305-348-1247, Fax 1-305-348-1986. tel. 1-310-383-2607. Associate Editor: Natalie Uhl, 228 Plant Science, Vice-Presidents: Jeff Brusseau, 1030 Heather Drive, Cornell University, Ithaca, New York 14853 USA, e- Vista, California 92084 USA, e-mail mail [email protected], tel. 1-607-257-0885. -

Index to Volume 60
PALM S Index to Vol. 60 Vol. 60(4) 2016 Index to Volume 60 A new species of Attalea from the Bolivian Attalea crassispatha 97, 113 lowlands 161 Attalea eichleri 111 A university palmetum 93 Attalea exigua 112 Acoelorrhaphe 97 Attalea huebneri 61, 69, 73, 74, 76, 114, Acoelorrhaphe wrightii 25–28, 97 117–119, 123 Acrocomia crispa 97, 113 Attalea insignis 76, 123 Adonidia dransfieldii 15 Attalea macrocarpa 122, 123 Adonidia merrillii 15, 97 Attalea maripa 59, 72, 74, 76 Aiphanes horrida 67, 70, 74, 113 Attalea moorei 58, 59, 62–64, 66–70, 72–74, Aiphanes minima 113 76, 117–121, 123 Aiphanes weberbaueri 72 Attalea osmantha 123 Andriamanantena, A.Z., as co-author 169 Attalea pacensis 162–165, 167 Ali, O.M.M.: The argun palm, Medemia Attalea peruviana 62, 64, 65, 77, 112, 122, argun , in the eastern Nubian Desert of 123 Sudan 145 Attalea phalerata 63, 68, 69, 73–76, 114, Allagoptera 111 117–120, 123, 162, 163, 165, 167 Areca 18 Attalea plowmanii 58, 62–64, 76, 110, 117, Areca catechu 3, 19 123 Arenga 17 Attalea polysticha 64, 65, 76, 112, 116 Arenga caudata 43 Attalea princeps 59, 71, 73, 76, 77, 118, 123, Arenga hookeriana 43 161, 162, 165, 167 Arenga pinnata 97 Attalea racemosa 62, 76 Arenga undulatifolia 97 Attalea rostrata 123 Aspects and causes of earlier and current Attalea salazarii 58, 61, 62 spread of Trachycarpus fortunei in the Attalea septuagenata 76 forests of southern Ticino and northern Attalea speciosa 76 Lago Maggiore (Switzerland, Italy) 125 Attalea tessmannii 59, 62, 64, 76, 113, 121, Astrocaryum 39, 113, 114 122 Astrocaryum carnosum 70 Attalea weberbaueri 59, 66, 67, 72, 73, 77, Astrocaryum faranae 70, 72 111, 112, 114, 119, 121, 123 Astrocaryum gratum 76 Attalea : Insights into the diversity and Astrocaryum huicungo 69 phylogeny of an intriguing genus 109 Astrocaryum perangustatum 72 Bactris 39 Astrocaryum ulei 74 Bactris gasipaes 39 Attalea 9, 11, 12, 18, 21, 39, 57–59, 63, 64, Bactris hirta 74 66, 69, 72, 74, 76, 77, 109–116, 121, Baker, W.J., W.L. -

Journal of the International Palm Society Vol. 58(4) Dec. 2014 the INTERNATIONAL PALM SOCIETY, INC
Palms Journal of the International Palm Society Vol. 58(4) Dec. 2014 THE INTERNATIONAL PALM SOCIETY, INC. The International Palm Society Palms (formerly PRINCIPES) Journal of The International Palm Society Founder: Dent Smith The International Palm Society is a nonprofit corporation An illustrated, peer-reviewed quarterly devoted to engaged in the study of palms. The society is inter- information about palms and published in March, national in scope with worldwide membership, and the June, September and December by The International formation of regional or local chapters affiliated with the Palm Society Inc., 9300 Sandstone St., Austin, TX international society is encouraged. Please address all 78737-1135 USA. inquiries regarding membership or information about Editors: John Dransfield, Herbarium, Royal Botanic the society to The International Palm Society Inc., 9300 Gardens, Kew, Richmond, Surrey, TW9 3AE, United Sandstone St., Austin, TX 78737-1135 USA, or by e-mail Kingdom, e-mail [email protected], tel. 44-20- to [email protected], fax 512-607-6468. 8332-5225, Fax 44-20-8332-5278. OFFICERS: Scott Zona, Dept. of Biological Sciences (OE 167), Florida International University, 11200 SW 8 Street, President: Leland Lai, 21480 Colina Drive, Topanga, Miami, Florida 33199 USA, e-mail [email protected], tel. California 90290 USA, e-mail [email protected], 1-305-348-1247, Fax 1-305-348-1986. tel. 1-310-383-2607. Associate Editor: Natalie Uhl, 228 Plant Science, Vice-Presidents: Jeff Brusseau, 1030 Heather Drive, Cornell University, Ithaca, New York 14853 USA, e- Vista, California 92084 USA, e-mail mail [email protected], tel. 1-607-257-0885. -
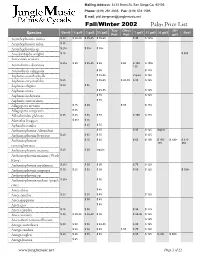
Winter-Fall Sale 2002 Palm Trees-Web
Mailing Address: 3233 Brant St. San Diego Ca, 92103 Phone: (619) 291 4605 Fax: (619) 574 1595 E mail: [email protected] Fall/Winter 2002 Palm Price List Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Acanthophoenix crinita $ 30 $ 30-40 $ 35-45 $ 55-65 $ 95 $ 125+ Acanthophoenix rubra $ 35 Acanthophoenix sp. $ 25+ $ 35+ $ 55+ Acoelorrhaphe wrightii $ 15 $ 300 Acrocomia aculeata $ 25+ $ 35 $ 35-45 $ 65 $ 65 $ 100- $ 150+ Actinokentia divaricata 135 Actinorhytis calapparia $ 55 $ 125 Aiphanes acanthophylla $ 45-55 inquire $ 125 Aiphanes caryotaefolia $ 25 $ 55-65 $ 45-55 $ 85 $ 125 Aiphanes elegans $ 20 $ 35 Aiphanes erosa $ 45-55 $ 125 Aiphanes lindeniana $ 55 $ 125 Aiphanes vincentsiana $ 55 Allagoptera arenaria $ 25 $ 40 $ 55 $ 135 Allagoptera campestris $ 35 Alloschmidtia glabrata $ 35 $ 45 $ 55 $ 85 $ 150 $ 175 Alsmithia longipes $ 35+ $ 55 Aphandra natalia $ 35 $ 55 Archontophoenix Alexandrae $ 55 $ 85 $ 125 inquire Archontophoenix Beatricae $ 20 $ 35 $ 55 $ 125 Archontophoenix $ 25 $ 45 $ 65 $ 100 $ 150- $ 200+ $ 310- 175 350 cunninghamiana Archontophoenix maxima $ 25 $ 30 inquire Archontophoenix maxima (Wash River) Archontophoenix myolaensis $ 25+ $ 30 $ 50 $ 75 $ 125 Archontophoenix purpurea $ 30 $ 25 $ 35 $ 50 $ 85 $ 125 $ 300+ Archontophoenix sp. Archontophoenix tuckerii (peach $ 25+ $ 55 river) Areca alicae $ 45 Areca catechu $ 20 $ 35 $ 45 $ 125 Areca guppyana $ 30 $ 45 Areca ipot $ 45 Areca triandra $ 25 $ 30 $ 95 $ 125 Areca vestiaria $ 25 $ 30-35 $ 35-40 $ 55 $ 85-95 $ 125 Arecastrum romanzoffianum $ 125 Arenga australasica $ 20 $ 30 $ 35 $ 45-55 $ 85 $ 125 Arenga caudata $ 20 $ 30 $ 45 $ 55 $ 75 $ 100 Arenga engleri $ 20 $ 60 $ 35 $ 45 $ 85 $ 125 $ 200 $ 300+ Arenga hastata $ 25 www.junglemusic.net Page 1 of 22 Tree Citrus 25/+ Band$ 1 gal$ 2 gal$ 3/5 gal$ 7 gal$ 15 gal$ 20 gal$ Box$ Species Pot$ Pot$ gal$ Arenga hookeriana inquire Arenga micranthe 'Lhutan' $ 20 inquire Arenga pinnata $ 35 $ 50 $ 85 $ 125 Arenga sp. -

Saving Tahina — You Can Help
July 2020 NEWSLETTER SAVING TAHINA — YOU CAN HELP PalmTalk (the public forum of the International Palm Society) is initiating a pro- gram to preserve Tahina spectabilis, an endangered palm that PalmTalk brought to the world’s attention in 2007. The discovery of this enormous, new palm gen- erated worldwide interest, but now due to its extremely limited and fragile range in Madagascar, Tahina needs our help. Please visit PalmTalk (go to www. palms.org, and click on the PalmTalk Forums tab) to see how the IPS and the Roy- al Botanic Gardens, Kew are partnering with a local village in Madagascar to help save this amazing palm. You can make a donation to the pro- ject and be a part of this unique partnership in conser- vation. Please donate today! One of the first photos posted on PalmTalk of the mysterious palm that came to be known as Tahina spectabilis and the little girl (at the time) for whom it was named, Anne-Tahina Metz. Volume 8.06 · July 2020 · Newsletter of the International Palm Society | Editor: Andy Hurwitz [email protected] Virtual hiking on Reunion Island, lowlands editionby Andy Hurwitz This article continues the “virtual hike” and tour of Reunion’s palms that began in last month’s News- letter. This month, we explore the palms and landscapes of the low to middle elevations. We begin by traveling to the windward eastern coast of the island, just south of Piton-Sainte-Rose, to ramble in Anse des Cascades. This idyllic cove is situated among groves of palm trees. The park is cool and shady. -

2951 – 3000 Trinidad to British Guiana
2951 – 3000 Trinidad to British Guiana [Inside cover] Book 9 Acanthophoenix 2956 Lecythis 2963 Acrocomia 2961 Licuala 2978 Ananas 2993 Livistona 2982 Archontophoenix 2983 Lodoicea 2985 Areca 2953 2953 Mauritia 2984 “ 2954 Mokka-mokka 2997 Astrocaryum 2957 Nipa 2981 “ 2986 Pachira 2976 “ 2987 “ 3000 Asystasia 2964 Passiflora 2952 Bauhinia 2960 “ 2995 Borassus 2979 Peltogyne 2970 Bromelia 2996 Pithecolobium 2965 Caryocar 2999 Randia 2994 Citrus 2998 Samanea 2966 Copernicia 2977 Undetermined 2967 Crotolaria 2973 “ In ink:]Ochna 2971 “ 2974 “ 2972 Desmoncus 2951 “ 2989 Diospyrus 2968 “ 2990 Euterpe 2955 “ 2991 Gmelina 2969 “ 2992 Hyphaene 2980 Zingerberaciae 2958 Ixora 2975 TILLANDSIA 2996 Jacaranda 2962 2951 Demoncus minor? See Harold Loomis’ notes and photo of inflorescence. #54 Villainously spacing & hooked climbing palm reminding one of those terrible Rattan palms of the Orient. When in fruit its bunches of deep scarlet fruits are attractive. A denizen of the deep shade forest and requiring moisture. From my experience in Coconut Grove with this genus I judge it wants half shade. Collected in Arena Forest Reserve Trinidad. 2/4/32 [In pencil] Loomis Photo 220 See D. F. Photo 18454-5 2952 P. H. Dorsett [In ink] “rubra?” Passiflora Sp Wild species of passion vine collected in the outskirts of the town of Eleuthera Bluff Island of Eleuthera Bahamas. Attractive looking species useful [Break in text, in ink] 1 single plant in pot Dec. 14/32. C. F. [Continuation of text] for breeding purposes. 1-7-32 (I’d like a few seeds or plants for my passiflora collection in Coconut Grove) Passiflora sp. Wild species growing in pothole in rocky soil of Eleuthera Bluff a colored town on Eleuthera Island. -

Species Delimitation and Hybrid Identification of Acrocomia Aculeata
Species delimitation and hybrid identification of Acrocomia aculeata and A. totai by genetic population approach Brenda D´ıaz1, Maria Zucchi2, Alessandro Alves-Pereira1, Joaquim Azevedo-Filho2, Mariana Sanit´a2, and Carlos Colombo2 1State University of Campinas 2Instituto Agronomico October 9, 2020 Abstract To the Neotropical genus Acrocomia (Arecaceae) is attributed eight species with a wide distribution in America. A. aculeata and A. totai are the most important species because of their high economic potential for oil production. However, there is no consensus in their classification as different taxons and their distinctiveness is particularly challenging due to morphological similarities with large plasticity of the traits. In addition, there is doubt about the occurrence of interspecific hybrids between both species. In this study, we applied a genetic population approach to assessing the genetic boundaries, diversity and to identify interspecific hybrids of A. aculeata and A. totai. Thirteen loci of simple sequence repeat (SSR) were employed to analyze twelve populations representing a wide distribution of species, from Minas Gerais, Brazil to Formosa, Argentina. Based on the Bayesian analysis (STRUCTURE and NewHybrids) and Discriminant Analysis of Principal Components (DAPC), our study supports the recognition of A. aculeata and A. totai as two species and the estimates of genetic parameters revealed more genetic diversity in A. totai (HE=0.551) than in A. aculeata (HE=0.466). We obtained evidence of hybridization between the species and that admixed individuals were assigned as F2 hybrids. In conclusion, this study showed the usefulness of microsatellite markers to elucidate the genetic boundaries of A. aculeata and A. totai, supporting their classification as different species and increase our knowledge about genetic diversity at the level of populations and species. -

Red Ring Disease of Coconut Palms Is Caused by the Red Ring Nematode (Bursaphelenchus Cocophilus), Though This Nematode May Also Be Known As the Coconut Palm Nematode
1 Red ring disease of coconut palms is caused by the red ring nematode (Bursaphelenchus cocophilus), though this nematode may also be known as the coconut palm nematode. This disease was first described on coconut palms in 1905 in Trinidad and the association between the disease and the nematode was reported in 1919. The vector of the nematode is the South American palm weevil (Rhynchophorus palmarum), both adults and larvae. The nematode parasitizes the weevil which then transmits the nematode as it moves from tree to tree. Though the weevil may visit many different tree species, the nematode only infects members of the Palmae family. The nematode and South American palm weevil have not yet been observed in Florida. 2 Information Sources: Brammer, A.S. and Crow, W.T. 2001. Red Ring Nematode, Bursaphelenchus cocophilus (Cobb) Baujard (Nematoda: Secernentea: Tylenchida: Aphelenchina: Aphelenchoidea: Bursaphelechina) formerly Rhadinaphelenchus cocophilus. University of Florida, IFAS Extension. EENY236. Accessed 11-27-13 http://edis.ifas.ufl.edu/in392 Griffith, R. 1987. “Red Ring Disease of Coconut Palm”. The American Pathological Society Plant Disease, Volume 71, February, 193-196. accessed 12/5/2013- http://www.apsnet.org/publications/plantdisease/ba ckissues/Documents/1987Articles/PlantDisease71n02_193.PDF Griffith, R., R. M. Giblin-Davis, P. K. Koshy, and V. K. Sosamma. 2005. Nematode parasites of coconut and other palms. M. Luc, R. A. Sikora, and J. Bridges (eds.) In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture. C.A.B. International, Oxon, UK. Pp. 493-527. 2 The host trees susceptible to the red ring nematode are usually found in the family Palmae. -

Monocotyledons and Gymnosperms of Puerto Rico and the Virgin Islands
SMITHSONIAN INSTITUTION Contributions from the United States National Herbarium Volume 52: 1-415 Monocotyledons and Gymnosperms of Puerto Rico and the Virgin Islands Editors Pedro Acevedo-Rodríguez and Mark T. Strong Department of Botany National Museum of Natural History Washington, DC 2005 ABSTRACT Acevedo-Rodríguez, Pedro and Mark T. Strong. Monocots and Gymnosperms of Puerto Rico and the Virgin Islands. Contributions from the United States National Herbarium, volume 52: 415 pages (including 65 figures). The present treatment constitutes an updated revision for the monocotyledon and gymnosperm flora (excluding Orchidaceae and Poaceae) for the biogeographical region of Puerto Rico (including all islets and islands) and the Virgin Islands. With this contribution, we fill the last major gap in the flora of this region, since the dicotyledons have been previously revised. This volume recognizes 33 families, 118 genera, and 349 species of Monocots (excluding the Orchidaceae and Poaceae) and three families, three genera, and six species of gymnosperms. The Poaceae with an estimated 89 genera and 265 species, will be published in a separate volume at a later date. When Ackerman’s (1995) treatment of orchids (65 genera and 145 species) and the Poaceae are added to our account of monocots, the new total rises to 35 families, 272 genera and 759 species. The differences in number from Britton’s and Wilson’s (1926) treatment is attributed to changes in families, generic and species concepts, recent introductions, naturalization of introduced species and cultivars, exclusion of cultivated plants, misdeterminations, and discoveries of new taxa or new distributional records during the last seven decades. -

Low-Maintenance Landscape Plants for South Florida1
ENH854 Low-Maintenance Landscape Plants for South Florida1 Jody Haynes, John McLaughlin, Laura Vasquez, Adrian Hunsberger2 Introduction regular watering, pruning, or spraying—to remain healthy and to maintain an acceptable aesthetic This publication was developed in response to quality. A low-maintenance plant has low fertilizer requests from participants in the Florida Yards & requirements and few pest and disease problems. In Neighborhoods (FYN) program in Miami-Dade addition, low-maintenance plants suitable for south County for a list of recommended landscape plants Florida must also be adapted to—or at least suitable for south Florida. The resulting list includes tolerate—our poor, alkaline, sand- or limestone-based over 350 low-maintenance plants. The following soils. information is included for each species: common name, scientific name, maximum size, growth rate An additional criterion for the plants on this list (vines only), light preference, salt tolerance, and was that they are not listed as being invasive by the other useful characteristics. Florida Exotic Pest Plant Council (FLEPPC, 2001), or restricted by any federal, state, or local laws Criteria (Burks, 2000). Miami-Dade County does have restrictions for planting certain species within 500 This section will describe the criteria by which feet of native habitats they are known to invade plants were selected. It is important to note, first, that (Miami-Dade County, 2001); caution statements are even the most drought-tolerant plants require provided for these species. watering during the establishment period. Although this period varies among species and site conditions, Both native and non-native species are included some general rules for container-grown plants have herein, with native plants denoted by †.