This Is an Open Access Document Downloaded from ORCA, Cardiff University's Institutional Repository
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity Patterns of the Vascular Plant Group Zosterophyllopsida in Relation to Devonian Paleogeography Borja Cascales-Miñana, Brigitte Meyer-Berthaud
Diversity patterns of the vascular plant group Zosterophyllopsida in relation to Devonian paleogeography Borja Cascales-Miñana, Brigitte Meyer-Berthaud To cite this version: Borja Cascales-Miñana, Brigitte Meyer-Berthaud. Diversity patterns of the vascular plant group Zosterophyllopsida in relation to Devonian paleogeography. Palaeogeography, Palaeoclimatology, Palaeoecology, Elsevier, 2015, 423, pp.53-61. 10.1016/j.palaeo.2015.01.024. hal-01140840 HAL Id: hal-01140840 https://hal-sde.archives-ouvertes.fr/hal-01140840 Submitted on 26 Nov 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Palaeogeography, Palaeoclimatology, Palaeoecology 423 (2015) 53–61 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Diversity patterns of the vascular plant group Zosterophyllopsida in relation to Devonian paleogeography Borja Cascales-Miñana a,b,⁎, Brigitte Meyer-Berthaud a a CNRS, Université de Montpellier, UMR Botanique et bioinformatique de l'architecture des plantes et des végétations (AMAP), F-34398 Montpellier Cedex 5, France b PPP, Département de Géologie, Université de Liège, Allée du 6 Août, B18 Sart Tilman, B-4000 Liège, Belgium article info abstract Article history: The Zosterophyllopsida originated in the Silurian and became prominent vascular components of Early Devonian Received 11 April 2014 floras worldwide. -

Fossils and Plant Phylogeny1
American Journal of Botany 91(10): 1683±1699. 2004. FOSSILS AND PLANT PHYLOGENY1 PETER R. CRANE,2,5 PATRICK HERENDEEN,3 AND ELSE MARIE FRIIS4 2Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK; 3Department of Biological Sciences, The George Washington University, Washington DC 20052 USA; 4Department of Palaeobotany, Swedish Museum of Natural History, Box 50007, S-104 05 Stockholm, Sweden Developing a detailed estimate of plant phylogeny is the key ®rst step toward a more sophisticated and particularized understanding of plant evolution. At many levels in the hierarchy of plant life, it will be impossible to develop an adequate understanding of plant phylogeny without taking into account the additional diversity provided by fossil plants. This is especially the case for relatively deep divergences among extant lineages that have a long evolutionary history and in which much of the relevant diversity has been lost by extinction. In such circumstances, attempts to integrate data and interpretations from extant and fossil plants stand the best chance of success. For this to be possible, what will be required is meticulous and thorough descriptions of fossil material, thoughtful and rigorous analysis of characters, and careful comparison of extant and fossil taxa, as a basis for determining their systematic relationships. Key words: angiosperms; fossils; paleobotany; phylogeny; spermatophytes; tracheophytes. Most biological processes, such as reproduction or growth distic context, neither fossils nor their stratigraphic position and development, can only be studied directly or manipulated have any special role in inferring phylogeny, and although experimentally using living organisms. Nevertheless, much of more complex models have been developed (see Fisher, 1994; what we have inferred about the large-scale processes of plant Huelsenbeck, 1994), these have not been widely adopted. -

Heterospory: the Most Iterative Key Innovation in the Evolutionary History of the Plant Kingdom
Biol. Rej\ (1994). 69, l>p. 345-417 345 Printeii in GrenI Britain HETEROSPORY: THE MOST ITERATIVE KEY INNOVATION IN THE EVOLUTIONARY HISTORY OF THE PLANT KINGDOM BY RICHARD M. BATEMAN' AND WILLIAM A. DiMlCHELE' ' Departments of Earth and Plant Sciences, Oxford University, Parks Road, Oxford OXi 3P/?, U.K. {Present addresses: Royal Botanic Garden Edinburiih, Inverleith Rojv, Edinburgh, EIIT, SLR ; Department of Geology, Royal Museum of Scotland, Chambers Street, Edinburgh EHi ijfF) '" Department of Paleohiology, National Museum of Natural History, Smithsonian Institution, Washington, DC^zo^bo, U.S.A. CONTENTS I. Introduction: the nature of hf^terospon' ......... 345 U. Generalized life history of a homosporous polysporangiophyle: the basis for evolutionary excursions into hetcrospory ............ 348 III, Detection of hcterospory in fossils. .......... 352 (1) The need to extrapolate from sporophyte to gametophyte ..... 352 (2) Spatial criteria and the physiological control of heterospory ..... 351; IV. Iterative evolution of heterospory ........... ^dj V. Inter-cladc comparison of levels of heterospory 374 (1) Zosterophyllopsida 374 (2) Lycopsida 374 (3) Sphenopsida . 377 (4) PtiTopsida 378 (5) f^rogymnospermopsida ............ 380 (6) Gymnospermopsida (including Angiospermales) . 384 (7) Summary: patterns of character acquisition ....... 386 VI. Physiological control of hetcrosporic phenomena ........ 390 VII. How the sporophyte progressively gained control over the gametophyte: a 'just-so' story 391 (1) Introduction: evolutionary antagonism between sporophyte and gametophyte 391 (2) Homosporous systems ............ 394 (3) Heterosporous systems ............ 39(1 (4) Total sporophytic control: seed habit 401 VIII. Summary .... ... 404 IX. .•Acknowledgements 407 X. References 407 I. I.NIRODUCTION: THE NATURE OF HETEROSPORY 'Heterospory' sensu lato has long been one of the most popular re\ie\v topics in organismal botany. -
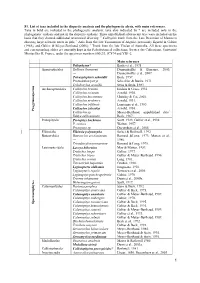
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

Structure, Development and Reproduction in Flowering Plants
Structure, Development and Reproduction in Flowering Plants Body Plan and Diversity in Form S.V.S Chauhan Professor Department of Botany B.R. Ambedkar University Khandari Campus Agra – 282002 [email protected] 1 Body Plan and Diversity in Form Every living organism has a fixed form and it is because of this reason that we are able to distinguish most of them just due to their external structure. Study of external morphology or external appearance of higher plants is necessary to describe the plants in an accurate fashion and to distinguish between almost similar looking plants. Therefore, the plants are identified by their morphological characters. Variation in plants is found not only in external forms but also in their anatomical characters which are represented by different types of tissue systems . Morphology along with anatomy constitute the base of studying pattern of life forms. Life Span of Plants On the basis of life span, plants are of three types: annuals, biennials and perennials. a) Annuals: These plants complete their life-cycle in a single growing season which varies from a few weeks to a few months. They pass the unfavourable period in the form of seeds. Examples are wheat, pea and sunflower, etc. b) Biennials: These plants complete their life-cycle in two growing seasons. In the first season; they grow only vegetatively and store food generally in the roots. In the second season, these plants grow at the expense of the stored food and form the flowering shoot bearing flowers, fruits and seeds. Then the plants die. radish, turnip, cabbage, etc. -

Plant Life MagillS Encyclopedia of Science
MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE Volume 4 Sustainable Forestry–Zygomycetes Indexes Editor Bryan D. Ness, Ph.D. Pacific Union College, Department of Biology Project Editor Christina J. Moose Salem Press, Inc. Pasadena, California Hackensack, New Jersey Editor in Chief: Dawn P. Dawson Managing Editor: Christina J. Moose Photograph Editor: Philip Bader Manuscript Editor: Elizabeth Ferry Slocum Production Editor: Joyce I. Buchea Assistant Editor: Andrea E. Miller Page Design and Graphics: James Hutson Research Supervisor: Jeffry Jensen Layout: William Zimmerman Acquisitions Editor: Mark Rehn Illustrator: Kimberly L. Dawson Kurnizki Copyright © 2003, by Salem Press, Inc. All rights in this book are reserved. No part of this work may be used or reproduced in any manner what- soever or transmitted in any form or by any means, electronic or mechanical, including photocopy,recording, or any information storage and retrieval system, without written permission from the copyright owner except in the case of brief quotations embodied in critical articles and reviews. For information address the publisher, Salem Press, Inc., P.O. Box 50062, Pasadena, California 91115. Some of the updated and revised essays in this work originally appeared in Magill’s Survey of Science: Life Science (1991), Magill’s Survey of Science: Life Science, Supplement (1998), Natural Resources (1998), Encyclopedia of Genetics (1999), Encyclopedia of Environmental Issues (2000), World Geography (2001), and Earth Science (2001). ∞ The paper used in these volumes conforms to the American National Standard for Permanence of Paper for Printed Library Materials, Z39.48-1992 (R1997). Library of Congress Cataloging-in-Publication Data Magill’s encyclopedia of science : plant life / edited by Bryan D. -

Earliest Record of Megaphylls and Leafy Structures, and Their Initial Diversification
Review Geology August 2013 Vol.58 No.23: 27842793 doi: 10.1007/s11434-013-5799-x Earliest record of megaphylls and leafy structures, and their initial diversification HAO ShouGang* & XUE JinZhuang Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, China Received January 14, 2013; accepted February 26, 2013; published online April 10, 2013 Evolutionary changes in the structure of leaves have had far-reaching effects on the anatomy and physiology of vascular plants, resulting in morphological diversity and species expansion. People have long been interested in the question of the nature of the morphology of early leaves and how they were attained. At least five lineages of euphyllophytes can be recognized among the Early Devonian fossil plants (Pragian age, ca. 410 Ma ago) of South China. Their different leaf precursors or “branch-leaf com- plexes” are believed to foreshadow true megaphylls with different venation patterns and configurations, indicating that multiple origins of megaphylls had occurred by the Early Devonian, much earlier than has previously been recognized. In addition to megaphylls in euphyllophytes, the laminate leaf-like appendages (sporophylls or bracts) occurred independently in several dis- tantly related Early Devonian plant lineages, probably as a response to ecological factors such as high atmospheric CO2 concen- trations. This is a typical example of convergent evolution in early plants. Early Devonian, euphyllophyte, megaphyll, leaf-like appendage, branch-leaf complex Citation: Hao S G, Xue J Z. Earliest record of megaphylls and leafy structures, and their initial diversification. Chin Sci Bull, 2013, 58: 27842793, doi: 10.1007/s11434- 013-5799-x The origin and evolution of leaves in vascular plants was phology and evolutionary diversification of early leaves of one of the most important evolutionary events affecting the basal euphyllophytes remain enigmatic. -

THE EVOLUTION of XYLEM ANATOMY in EARLY TRACHEOPHYTES by ELISABETH ANNE BERGMAN
Conquering the terrestrial environment: the evolution of xylem anatomy in early tracheophytes Item Type text; Electronic Thesis Authors Bergman, Elisabeth Anne Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 27/09/2021 03:01:29 Item License http://rightsstatements.org/vocab/InC/1.0/ Link to Item http://hdl.handle.net/10150/626731 CONQUERING THE TERRESTRIAL ENVIRONMENT: THE EVOLUTION OF XYLEM ANATOMY IN EARLY TRACHEOPHYTES By ELISABETH ANNE BERGMAN ____________________ A Thesis Submitted to The Honors College In Partial Fulfillment of the Bachelors Degree With Honors in Biology with an Emphasis in Biomedical Sciences THE UNIVERSITY OF ARIZONA D E C E M B E R 2 0 1 7 Approved by: ____________________________ Dr. Brian Enquist Department of Ecology and Evolutionary Biology Acknowledgements Many thanks go to all of those who made contributions, big and small, to my honors thesis, and more notably, my education. Foremost, I thank Dr. Brian Enquist for accepting me into his lab and serving as my mentor for two years. I appreciate all of the time he put in to meet with me and help me to develop my honors thesis. Additional thanks go to Dr. Sean Michaletz who first introduced me to the work that would eventually become my honors thesis. From the University of Santa Cruz, California, I thank Dr. -

Additional Observations on Zosterophyllum Yunnanicum Hsü from the Lower Devonian of Yunnan, China
This is an Open Access document downloaded from ORCA, Cardiff University's institutional repository: http://orca.cf.ac.uk/77818/ This is the author’s version of a work that was submitted to / accepted for publication. Citation for final published version: Edwards, Dianne, Yang, Nan, Hueber, Francis M. and Li, Cheng-Sen 2015. Additional observations on Zosterophyllum yunnanicum Hsü from the Lower Devonian of Yunnan, China. Review of Palaeobotany and Palynology 221 , pp. 220-229. 10.1016/j.revpalbo.2015.03.007 file Publishers page: http://dx.doi.org/10.1016/j.revpalbo.2015.03.007 <http://dx.doi.org/10.1016/j.revpalbo.2015.03.007> Please note: Changes made as a result of publishing processes such as copy-editing, formatting and page numbers may not be reflected in this version. For the definitive version of this publication, please refer to the published source. You are advised to consult the publisher’s version if you wish to cite this paper. This version is being made available in accordance with publisher policies. See http://orca.cf.ac.uk/policies.html for usage policies. Copyright and moral rights for publications made available in ORCA are retained by the copyright holders. @’ Additional observations on Zosterophyllum yunnanicum Hsü from the Lower Devonian of Yunnan, China Dianne Edwardsa, Nan Yangb, Francis M. Hueberc, Cheng-Sen Lib a*School of Earth and Ocean Sciences, Cardiff University, Park Place, Cardiff CF10 3AT, UK b Institute of Botany, Chinese Academy of Sciences, Beijing 100093, China cNational Museum of Natural History, Smithsonian Institution, Washington D.C. 20560-0121, USA * Corresponding author, Tel.: +44 29208742564, Fax.: +44 2920874326 E-mail address: [email protected] ABSTRACT Investigation of unfigured specimens in the original collection of Zosterophyllum yunnanicum Hsü 1966 from the Lower Devonian (upper Pragian to basal Emsian) Xujiachong Formation, Qujing District, Yunnan, China has provided further data on both sporangial and stem anatomy. -

University of Michigan University Library
CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY , THE UNIVERSITY OF MICHIGAN VOL. XWI, No. 11, pp. 241-263 (2 pk., 5 figs.) OCTOBER9, 1962 A MISSISSIPPIAN FLORA FROM NORTHEASTERN UTAH AND ITS FAUNAL AND STRATIGRAPHIC RELATIONS BY CHESTER A. ARNOLD and WALTER SADLICK Published with aid from the Edward Pulteney Wright and Jean Davies Wright Expendable Trust Fund MUSEUM OF PfiEONTOLOGY THE UNIVERSITY OF MICHIGAN ANN ARBOR CONTRIBUTIONS FROM THE MUSEUM OF PALEONTOLOGY Director: LEWISB. KELLUM The series of contributions from the Museum of Paleontology is a medium for the publication of papers based chiefly upon the collection in the Museum. When the number of pages issued is sufficient to make a volume, a title page and a table of contents will be sent to libraries on the mailing list, and to individuals upon request. A list of the separate papers may also be obtained. Correspondence should be directed to the Museum of Paleontology, The University of Michigan, Ann Arbor, Michigan. VOLS.11-XV. Parts of volumes may be obtained if available. VOLUMEXVI 1. Two Late Pleistocene Faunas from Southwestern Kansas, by Claude W. Hibbard and Dwight W. Taylor. Pages 1-223, with 16 plates. 2. North American Genera of the Devonian Rugose Coral Family Digonophylli- dae, by Erwin C. Stumm. Pages 225-243, with 6 plates. 3. Notes on Jaekelocystis hartleyi and Pseudocrinjtes gordoni, two Rhombi- feran Cystoids Described by Charles Schuchert in 1903, by Robert V. Kesling. Pages 245-273, with 8 plates. 4. Corals of the Traverse Group of Michigan. Part VI, Cladopora, Striatopora, and Thamnopora, by Erwin C. -

Please Scroll Down for Article
This article was downloaded by: [Retallack, Gregory J.] On: 2 November 2009 Access details: Access Details: [subscription number 916429953] Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Alcheringa: An Australasian Journal of Palaeontology Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t770322720 Cambrian-Ordovician non-marine fossils from South Australia Gregory J. Retallack a a Department of Geological Sciences, University of Oregon, Eugene, OR, USA Online Publication Date: 01 December 2009 To cite this Article Retallack, Gregory J.(2009)'Cambrian-Ordovician non-marine fossils from South Australia',Alcheringa: An Australasian Journal of Palaeontology,33:4,355 — 391 To link to this Article: DOI: 10.1080/03115510903271066 URL: http://dx.doi.org/10.1080/03115510903271066 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden. The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material. -

Belowground Rhizomes in Paleosols: the Hidden Half of an Early Devonian Vascular Plant
Belowground rhizomes in paleosols: The hidden half of an Early Devonian vascular plant Jinzhuang Xuea,b,1, Zhenzhen Denga, Pu Huanga, Kangjun Huanga, Michael J. Bentonc, Ying Cuid, Deming Wanga, Jianbo Liua, Bing Shena, James F. Basingere, and Shougang Haoa aThe Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, People’s Republic of China; bKey Laboratory of Economic Stratigraphy and Palaeogeography, Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing 210008, People’s Republic of China; cSchool of Earth Sciences, University of Bristol, Bristol BS8 1RJ, United Kingdom; dDepartment of Earth Sciences, Dartmouth College, Hanover, NH 03755; and eDepartment of Geological Sciences, University of Saskatchewan, Saskatoon, SK S7N 5E2, Canada Edited by Donald E. Canfield, Institute of Biology and Nordic Center for Earth Evolution, University of Southern Denmark, Odense M., Denmark, and approved June 28, 2016 (received for review March 28, 2016) The colonization of terrestrial environments by rooted vascular because of a poor fossil record, it remains quite unclear how the plants had far-reaching impacts on the Earth system. However, “hidden half” ecosystem (24), with buried structures growing in soils, the belowground structures of early vascular plants are rarely functioned during the early stage of vascular plant radiation. Such a documented, and thus the plant−soil interactions in early terres- knowledge gap hinders a deep understanding of the ecology of early trial ecosystems are poorly understood. Here we report the earli- plants and their roles in terrestrial environments. est rooted paleosols (fossil soils) in Asia from Early Devonian In this article, we report well-preserved plant traces from the deposits of Yunnan, China.